HPLC-based cytotoxicity profiling and LC-ESIQTOF-MS/MS analysis of Helichrysum leucocephalum
- PMID: 38449622
- PMCID: PMC10915411
- DOI: 10.1016/j.heliyon.2024.e27230
HPLC-based cytotoxicity profiling and LC-ESIQTOF-MS/MS analysis of Helichrysum leucocephalum
Abstract
Introduction: Helichrysum leucocephalum Boiss. (Asteraceae) is an endemic plant to Iran. No reports have studied the cytotoxicity of the plant. The current study aimed to evaluate the cytotoxicity of H. leucocephalum collected from Fars province (Iran) against MCF-7 and HDF cell lines using HPLC-based activity profiling and to annotate the active constituents by LC-ESIQTOF-MS/MS.
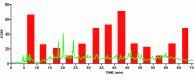
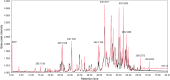
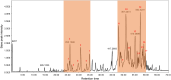
Methods: H. leucocephalum was collected from three locations in Fars province. The dried flowers and leaves were separately extracted by percolation using methanol. The crude extracts were fractionated by liquid-liquid partitioning with dichloromethane (DCM) and aqueous methanol. The cytotoxicity of the fractions was evaluated against MCF-7 and HDF cells by Alamarblue assay. HPLC-based activity profiling was used to track the active constituents. LC-MS dereplication strategy was used for the annotation of the compounds in the active time window. LC-MS data were preprocessed by MZmine 3.3.0 and submitted to multivariate analysis to compare the differences and similarities in the metabolites of the samples.
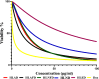
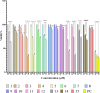
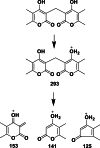
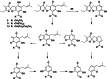
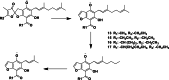
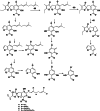
Results: The DCM fractions showed a dose-dependent cytotoxicity against the cancerous cells (IC50s, 9.8-105.1 μg/ml). In general, the metabolites of the flowers and their cytotoxicity were higher than the leaves. LCESIMS/MS analyses revealed that prenylated and geranylated α,β-unsaturated spiroketal phloroglucinols were among the active constituents.
Conclusion: It can be concluded that H. leucocephalum is a rich source of phloroglucinol derivatives with cytotoxic activities. Further phytochemical analysis is needed to characterize the bioactive components.
Keywords: Asteraceae; Cytotoxicity; HPLC; Helichrysum leucocephalum; LC-ESIQTOF-MS/MS.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









Similar articles
-
Extraction, fractionation and re-fractionation of Artemisia nilagirica for anticancer activity and HPLC-ESI-QTOF-MS/MS determination.J Ethnopharmacol. 2018 Mar 1;213:72-80. doi: 10.1016/j.jep.2017.10.029. J Ethnopharmacol. 2018. PMID: 29109061
-
LC-MS/MS-Based Metabolomic Profiling of Constituents from Glochidion velutinum and Its Activity against Cancer Cell Lines.Molecules. 2022 Dec 17;27(24):9012. doi: 10.3390/molecules27249012. Molecules. 2022. PMID: 36558144 Free PMC article.
-
In vitro antitumor actions of extracts from endemic plant Helichrysum zivojinii.BMC Complement Altern Med. 2013 Feb 18;13:36. doi: 10.1186/1472-6882-13-36. BMC Complement Altern Med. 2013. PMID: 23414290 Free PMC article.
-
Phytochemicals and Cytotoxicity of Launaea procumbens on Human Cancer Cell Lines.Pharmacogn Mag. 2016 Jul;12(Suppl 4):S431-S435. doi: 10.4103/0973-1296.191452. Pharmacogn Mag. 2016. PMID: 27761070 Free PMC article.
-
Helichrysum italicum: from traditional use to scientific data.J Ethnopharmacol. 2014;151(1):54-65. doi: 10.1016/j.jep.2013.11.005. Epub 2013 Nov 14. J Ethnopharmacol. 2014. PMID: 24239849 Review.
References
-
- Akaberi M., Sahebkar A., Azizi N., Emami S.A. Everlasting flowers: phytochemistry and pharmacology of the genus Helichrysum. Ind. Crops Prod. 2019;138 doi: 10.1016/j.indcrop.2019.111471. - DOI
-
- Ghahreman A. Iran University Press; Tehran: 1994. Iranian Choromophites (Botanical Systematic)
-
- Mastelic J., Politeo O., Jerkovic I., Radosevic N. Composition and antimicrobial activity of Helichrysum italicum essential oil and its terpene and terpenoid fractions. Chem. Nat. Compd. 2005;41(1):35–40.
LinkOut - more resources
Full Text Sources

