Efficacy, tolerability and safety of add-on third-generation antiseizure medications in treating focal seizures worldwide: a network meta-analysis of randomised, placebo-controlled trials
- PMID: 38449838
- PMCID: PMC10915785
- DOI: 10.1016/j.eclinm.2024.102513
Efficacy, tolerability and safety of add-on third-generation antiseizure medications in treating focal seizures worldwide: a network meta-analysis of randomised, placebo-controlled trials
Abstract
Background: Adjunctive newer antiseizure medications (ASMs) are being used in patients with treatment-resistant focal-onset seizures (FOS). An updated network meta-analysis (NMA) was necessary to compile evidence in this critical area.
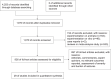
Methods: We systematically searched PubMed, Embase, Cochrane Library, Web of Science, and Scopus from their inception until 17 January 2024, evaluating the efficacy, tolerability, and safety of rufinamide (RUF), brivaracetam (BRV), cenobamate (CNB), eslicarbazepine (ESL), lacosamide (LCM), retigabine (RTG), and perampanel (PER) as adjunctive treatments for FOS. Efficacy outcomes included seizure response and seizure freedom. Tolerability was assessed by discontinuation due to adverse events (AEs). Safety outcomes were evaluated based on the number of patients experiencing at least one AE and serious adverse events (SAEs). This review is registered with PROSPERO (CRD42023485130).
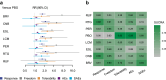
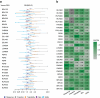
Findings: A total of 29 studies involving 11,750 participants were included. For seizure response, all ASMs were significantly superior to placebo, with RTG ranking highest, followed by CNB. Considering dosage, CNB 400 mg/d was top-ranked, followed by RTG 1200 mg/d. For seizure freedom, BRV was highest-ranked, followed by CNB, with BRV 100 mg/d leading, followed by CNB 400 mg/d. Regarding tolerability, LCM 600 mg/d had the lowest ranking, followed by CNB 400 mg/d. For the safety outcome of AEs, ESL 1200 mg/d was ranked lowest, followed by CNB 400 mg/d. Regarding SAEs, LCM 400 mg/d was ranked lowest, followed by RTG 1200 mg/d.
Interpretation: ASMs at different dosages have varying efficacy and tolerability profiles. We have provided hierarchical rankings of ASMs for efficacy and safety outcomes. Our findings offer the most comprehensive evidence available to inform patients, families, physicians, guideline developers, and policymakers about the choice of ASMs in patients with treatment-resistant FOS.
Funding: None.
Keywords: Antiseizure medications; Epilepsy; Focal seizures; Network meta-analysis; Seizure.
© 2024 The Author(s).
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Efficacy and safety of add-on antiseizure medications for focal epilepsy: A network meta-analysis.Epilepsia Open. 2024 Aug;9(4):1550-1564. doi: 10.1002/epi4.12997. Epub 2024 Jun 18. Epilepsia Open. 2024. PMID: 38888005 Free PMC article.
-
The efficacy and safety of third-generation antiseizure medications and non-invasive brain stimulation to treat refractory epilepsy: a systematic review and network meta-analysis study.Front Neurol. 2024 Jan 9;14:1307296. doi: 10.3389/fneur.2023.1307296. eCollection 2023. Front Neurol. 2024. PMID: 38264091 Free PMC article.
-
Efficacy and tolerability of brivaracetam compared to lacosamide, eslicarbazepine acetate, and perampanel as adjunctive treatments in uncontrolled focal epilepsy: Results of an indirect comparison meta-analysis of RCTs.Seizure. 2016 Nov;42:29-37. doi: 10.1016/j.seizure.2016.08.007. Epub 2016 Sep 24. Seizure. 2016. PMID: 27710868 Review.
-
Efficacy and Tolerability of Adjunctive Brivaracetam in Patients with Focal-Onset Seizures on Specific Concomitant Antiseizure Medications: Pooled Analysis of Double-Blind, Placebo-Controlled Trials.Adv Ther. 2024 Apr;41(4):1746-1758. doi: 10.1007/s12325-024-02795-z. Epub 2024 Feb 15. Adv Ther. 2024. PMID: 38356105 Free PMC article. Clinical Trial.
-
Retention, efficacy, tolerability, and quality of life during long-term adjunctive brivaracetam treatment by number of lifetime antiseizure medications: A post hoc analysis of phase 3 trials in adults with focal seizures.Epilepsy Behav. 2023 Jan;138:108967. doi: 10.1016/j.yebeh.2022.108967. Epub 2022 Nov 23. Epilepsy Behav. 2023. PMID: 36435010 Clinical Trial.
Cited by
-
Efficacy and safety of add-on antiseizure medications for focal epilepsy: A network meta-analysis.Epilepsia Open. 2024 Aug;9(4):1550-1564. doi: 10.1002/epi4.12997. Epub 2024 Jun 18. Epilepsia Open. 2024. PMID: 38888005 Free PMC article.
-
Cenobamate: A Review in Focal-Onset Seizures.CNS Drugs. 2025 Jul;39(7):707-719. doi: 10.1007/s40263-025-01178-4. Epub 2025 Apr 14. CNS Drugs. 2025. PMID: 40227505 Review.
-
Long-Term Safety and Efficacy of Lacosamide Combined with NOACs in Post-Stroke Epilepsy and Atrial Fibrillation: A Prospective Longitudinal Study.J Pers Med. 2024 Nov 27;14(12):1125. doi: 10.3390/jpm14121125. J Pers Med. 2024. PMID: 39728038 Free PMC article.
-
Adverse effects of antiseizure medications: a review of the impact of pharmacogenetics and drugs interactions in clinical practice.Front Pharmacol. 2025 Jul 10;16:1584566. doi: 10.3389/fphar.2025.1584566. eCollection 2025. Front Pharmacol. 2025. PMID: 40709084 Free PMC article. Review.
References
-
- World Health Organization . 2012. Fact sheets-epilepsy.http://www.who.int/mediacentre/factsheets/fs999/en/index.html Accessed 27 Feb 2013.
LinkOut - more resources
Full Text Sources

