Safety and Immune Responses Following Anti-PD-1 Monoclonal Antibody Infusions in Healthy Persons With Human Immunodeficiency Virus on Antiretroviral Therapy
- PMID: 38449916
- PMCID: PMC10917183
- DOI: 10.1093/ofid/ofad694
Safety and Immune Responses Following Anti-PD-1 Monoclonal Antibody Infusions in Healthy Persons With Human Immunodeficiency Virus on Antiretroviral Therapy
Abstract
Background: T cells in people with human immunodeficiency virus (HIV) demonstrate an exhausted phenotype, and HIV-specific CD4+ T cells expressing programmed cell death 1 (PD-1) are enriched for latent HIV, making antibody to PD-1 a potential strategy to target the latent reservoir.
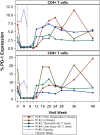
Methods: This was a phase 1/2, randomized (4:1), double-blind, placebo-controlled study in adults with suppressed HIV on antiretroviral therapy with CD4+ counts ≥350 cells/μL who received 2 infusions of cemiplimab versus placebo. The primary outcome was safety, defined as any grade 3 or higher adverse event (AE) or any immune-related AE (irAE). Changes in HIV-1-specific polyfunctional CD4+ and CD8+ T-cell responses were evaluated.
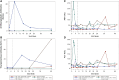
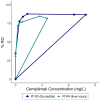
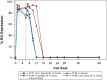
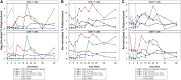
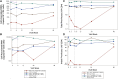
Results: Five men were enrolled (median CD4+ count, 911 cells/μL; median age, 51 years); 2 received 1 dose of cemiplimab, 2 received 2 doses, and 1 received placebo. One participant had a probable irAE (thyroiditis, grade 2); another had a possible irAE (hepatitis, grade 3), both after a single low-dose (0.3 mg/kg) infusion. The Safety Monitoring Committee recommended no further enrollment or infusions. All 4 cemiplimab recipients were followed for 48 weeks. No other cemiplimab-related serious AEs, irAEs, or grade 3 or higher AEs occurred. One 2-dose recipient of cemiplimab had a 6.2-fold increase in polyfunctional, Gag-specific CD8+ T-cell frequency with supportive increases in plasma HIV RNA and decreases in total HIV DNA.
Conclusions: One of 4 participants exhibited increased HIV-1-specific T-cell responses and transiently increased HIV-1 expression following 2 cemiplimab infusions. The occurrence of irAEs after a single, low dose may limit translating the promising therapeutic results of cemiplimab for cancer to immunotherapeutic and latency reversal strategies for HIV. Clinical Trials Registration. NCT03787095.
Keywords: HIV; HIV cure; HIV latency; anti-PD-1 inhibitor; immune checkpoint inhibitors.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. L. G. receives research support from Gilead Sciences, ViiV Healthcare, Moderna, and Novavax. D. R. K. has received research support and/or consulting honoraria from AbbVie, Gilead, GlaxoSmithKline, Janssen, Merck, and ViiV. B. J. M. has received research support from AstraZeneca. A. P., V. J., and E. M. are employees of Regeneron Pharmaceuticals. W. D. H. has served as a consultant for Enochian Biosciences, Gilead, Merck, and ViiV/GSK. J. J. E. receives research support from ViiV Healthcare and Gilead Sciences and consulting honoraria from ViiV, Gilead Sciences, and Merck. C. A. B. receives research support from Gilead Sciences. E. T. O. took a position with ViiV Healthcare after the completion of this work. All other authors report no potential conflicts.
Figures
References
-
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342:1432–3. - PubMed
-
- Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006; 12:1198–202. - PubMed
-
- Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492–9. - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

