Effects of flusulfamide on spore germination of Plasmodiophora brassicae
- PMID: 38450088
- PMCID: PMC10912929
- DOI: 10.1584/jpestics.D23-031
Effects of flusulfamide on spore germination of Plasmodiophora brassicae
Abstract
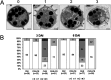
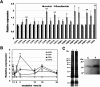
Flusulfamide inhibits germination of Plasmodiophora brassicae resting spores to suppress clubroot disease, but its mechanism of action on the germination of P. brassicae resting spores remains unclear. In this study, P. brassicae resting spores were treated with flusulfamide and visualized using transmission electron microscopy (TEM). The gene expression of P. brassicae resting spores was analyzed using RT-PCR, followed by immunoblotting analysis. TEM results revealed that flusulfamide suppressed the primary zoosporogenesis of P. brassicae resting spores during the early phase, and RT-PCR results revealed that flusulfamide affected the gene expression during the germination of the resting spores. Immunoblot and RT-qPCR analyses revealed that PbCyp3, an immunophilin (peptidyl-prolyl-isomerase) gene, was highly expressed, resulting in the unusual accumulation of PbCYP3 protein in P. brassicae resting spores immediately after treatment with flusulfamide. This suggests that flusulfamide may cause aberrant folding of proteins involved in primary zoosporogenesis, thereby inhibiting germination.
Keywords: clubroot; flusulfamide; immunophilin; primary zoosporogenesis; resting spores.
© 2024 Pesticide Science Society of Japan.
Figures
References
-
- 1) G. D. Dixon: Clubroot (Plasmodiophora brassicae Woronin)-an agricultural and biological challenge worldwide. Can. J. Plant Pathol. 36 (sup1), 5–18 (2014).
-
- 2) J. S. Karling: “The Plasmodiophorales: Including a Complete Host Index, Bibliography, and a Description of Diseases Caused by Species of This Order,” Hafner Publishing, New York, 1968.
-
- 3) S. Tanaka, S. Yoshihara, S. Ito and M. Kameya-Iwaki: The influence of virulence of Plasmodiophora brassicae populations on epidemiology of Chinese cabbage clubroot and efficacy of fungicides. Ann. Phytopathol. Soc. Jpn. 63, 183–187 (1997), in Japanese.
-
- 4) L. S. Kowata-Dresch and L. L. May-De Mio: Clubroot management of highly infested soils. Crop Prot. 35, 47–52 (2012).
-
- 5) S. Tanaka, S. Kochi, H. Kunita, S. Ito and M. Kameya-Iwaki: Biological mode of action of the fungicide, flusulfamide, against Plasmodiophora brassicae (clubroot). Eur. J. Plant Pathol. 105, 577–584 (1999).

