Pseudouridine synthase 1 regulates erythropoiesis via transfer RNAs pseudouridylation and cytoplasmic translation
- PMID: 38450158
- PMCID: PMC10915626
- DOI: 10.1016/j.isci.2024.109265
Pseudouridine synthase 1 regulates erythropoiesis via transfer RNAs pseudouridylation and cytoplasmic translation
Abstract
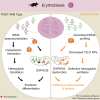
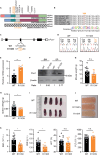
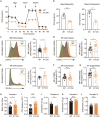
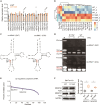
Pseudouridylation plays a regulatory role in various physiological and pathological processes. A prime example is the mitochondrial myopathy, lactic acidosis, and sideroblastic anemia syndrome (MLASA), characterized by defective pseudouridylation resulting from genetic mutations in pseudouridine synthase 1 (PUS1). However, the roles and mechanisms of pseudouridylation in normal erythropoiesis and MLASA-related anemia remain elusive. We established a mouse model carrying a point mutation (R110W) in the enzymatic domain of PUS1, mimicking the common mutation in human MLASA. Pus1-mutant mice exhibited anemia at 4 weeks old. Impaired mitochondrial oxidative phosphorylation was also observed in mutant erythroblasts. Mechanistically, mutant erythroblasts showed defective pseudouridylation of targeted tRNAs, altered tRNA profiles, decreased translation efficiency of ribosomal protein genes, and reduced globin synthesis, culminating in ineffective erythropoiesis. Our study thus provided direct evidence that pseudouridylation participates in erythropoiesis in vivo. We demonstrated the critical role of pseudouridylation in regulating tRNA homeostasis, cytoplasmic translation, and erythropoiesis.
Keywords: Cell biology; Molecular biology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Mitochondrial tRNA pseudouridylation governs erythropoiesis.Blood. 2024 Aug 8;144(6):657-671. doi: 10.1182/blood.2023022004. Blood. 2024. PMID: 38635773
-
Mitochondrial myopathy and sideroblastic anemia (MLASA): missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation.J Biol Chem. 2005 May 20;280(20):19823-8. doi: 10.1074/jbc.M500216200. Epub 2005 Mar 16. J Biol Chem. 2005. PMID: 15772074
-
Pleiotropic effects and compensation mechanisms determine tissue specificity in mitochondrial myopathy and sideroblastic anemia (MLASA).Mol Genet Metab. 2007 Jun;91(2):148-56. doi: 10.1016/j.ymgme.2007.02.006. Epub 2007 Mar 19. Mol Genet Metab. 2007. PMID: 17374500 Free PMC article.
-
A Novel PUS1 Mutation in 2 Siblings with MLASA Syndrome: A Review of the Literature.J Pediatr Hematol Oncol. 2021 May 1;43(4):e592-e595. doi: 10.1097/MPH.0000000000001806. J Pediatr Hematol Oncol. 2021. PMID: 32287105 Review.
-
Research progress of RNA pseudouridine modification in nervous system.Int J Neurosci. 2025 Jun;135(6):639-649. doi: 10.1080/00207454.2024.2315483. Epub 2024 Feb 29. Int J Neurosci. 2025. PMID: 38407188 Review.
Cited by
-
Mitochondrial diseases: from molecular mechanisms to therapeutic advances.Signal Transduct Target Ther. 2025 Jan 10;10(1):9. doi: 10.1038/s41392-024-02044-3. Signal Transduct Target Ther. 2025. PMID: 39788934 Free PMC article. Review.
-
Functions and therapeutic applications of pseudouridylation.Nat Rev Mol Cell Biol. 2025 May 20. doi: 10.1038/s41580-025-00852-1. Online ahead of print. Nat Rev Mol Cell Biol. 2025. PMID: 40394244 Review.
-
RNA modifications in cancer.MedComm (2020). 2025 Jan 10;6(1):e70042. doi: 10.1002/mco2.70042. eCollection 2025 Jan. MedComm (2020). 2025. PMID: 39802639 Free PMC article. Review.
References
-
- Cohn W.E. Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: isolation, structure, and chemical characteristics. J. Biol. Chem. 1960;235:1488–1498. - PubMed
-
- Machnicka M.A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M., et al. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. - DOI - PMC - PubMed
-
- Guzzi N., Muthukumar S., Cieśla M., Todisco G., Ngoc P.C.T., Madej M., Munita R., Fazio S., Ekström S., Mortera-Blanco T., et al. Pseudouridine-modified tRNA fragments repress aberrant protein synthesis and predict leukaemic progression in myelodysplastic syndrome. Nat. Cell Biol. 2022;24:299–306. doi: 10.1038/s41556-022-00852-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

