Oral Clostridium butyricum on mice endometritis through uterine microbiome and metabolic alternations
- PMID: 38450161
- PMCID: PMC10915095
- DOI: 10.3389/fmicb.2024.1351899
Oral Clostridium butyricum on mice endometritis through uterine microbiome and metabolic alternations
Abstract
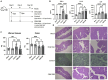
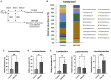
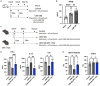
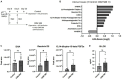
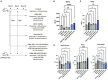
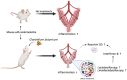
Endometritis occurs frequently in humans and animals, which can negatively affect fertility and cause preterm parturition syndrome. Orally administered Clostridium butyricum, a butyrate-producing gram-positive anaerobe, exhibits anti-inflammatory effects. However, the precise mechanism by which Clostridium butyricum attenuates endometritis remains unclear. This in vivo study evaluated the anti-inflammatory effects of orally administered Clostridium butyricum on uterine tissues. In addition, we conducted uterine microbiome and lipid metabolome analyses to determine the underlying mechanisms. Female Balb/c mice were divided into the following four groups (n = 5-20): (1) mock group, (2) only operation group (mice only underwent operation to exposed uterine horns from the side), (3) control group (mice underwent the same operation with the operation group + perfusion of lipopolysaccharide solution from uterine horns), and (4) Clostridium butyricum administration group (mice underwent the same operation with the control group + oral Clostridium butyricum administration from days 0 to 9). Clostridium butyricum was administered via oral gavage. On day 10, we investigated protein expression, uterine microbiome, and lipid metabolism in uterine tissues. Consequently, orally administered Clostridium butyricum altered the uterine microbiome and induced proliferation of Lactobacillus and Limosilactobacillus species. The effects can contribute to show the anti-inflammatory effect through the interferon-β upregulation in uterine tissues. Additionally, oral Clostridium butyricum administration resulted in the upregulations of some lipid metabolites, such as ω-3 polyunsaturated fatty acid resolvin D5, in uterine tissues, and resolvin D5 showed anti-inflammatory effects. However, the orally administered Clostridium butyricum induced anti-inflammatory effect was attenuated with the deletion of G protein-coupled receptor 120 and 15-lipooxgenase inhibition. In conclusion, Clostridium butyricum in the gut has anti-inflammatory effects on uterine tissues through alterations in the uterine microbiome and lipid metabolism. This study revealed a gut-uterus axis mechanism and provided insights into the treatment and prophylaxis of endometritis.
Keywords: Clostridium butyricum; G protein-coupled receptor 120; Lactobacillus species; Limosilactobacillus species; endometritis; metabolome; microbiome; resolvin D5.
Copyright © 2024 Hagihara, Ariyoshi, Eguchi, Oka, Takahashi, Kato, Shibata, Umemura, Mori, Miyazaki, Hirai, Asai, Mori and Mikamo.
Conflict of interest statement
TA, SE, KO, and MT are employees of Miyarisan Pharmaceutical Co., Ltd. HM received research funding and Ltd. consulting fee/honorarium from Miyarisan Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Ariyoshi T., Hagihara M., Eguchi S., Fukuda A., Iwasaki K., Oka K., et al. (2020). Clostridium butyricum MIYAIRI 588-induced Protectin D1 has an anti-inflammatory effect on antibiotic-induced intestinal disorder. Front. Microbiol. 11:587725. doi: 10.3389/fmicb.2020.587725, PMID: - DOI - PMC - PubMed
-
- Ariyoshi T., Hagihara M., Tomono S., Eguchi S., Minemura A., Miura D., et al. (2021). Clostridium butyricum MIYAIRI 588 modifies bacterial composition under antibiotic-induced dysbiosis for the activation of interactions via lipid metabolism between the gut microbiome and the host. Biomedicines 9:1065. doi: 10.3390/biomedicines9081065, PMID: - DOI - PMC - PubMed
-
- Bourgeois C., Majer O., Frohner I. E., Lesiak-Markowicz I., Hildering K.-S., Glaser W., et al. (2011). Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-β signaling. J. Immunol. 186, 3104–3112. doi: 10.4049/jimmunol.1002599, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

