BioPrev-C - development and validation of a contemporary prostate cancer risk calculator
- PMID: 38450183
- PMCID: PMC10915644
- DOI: 10.3389/fonc.2024.1343999
BioPrev-C - development and validation of a contemporary prostate cancer risk calculator
Abstract
Objectives: To develop a novel biopsy prostate cancer (PCa) prevention calculator (BioPrev-C) using data from a prospective cohort all undergoing mpMRI targeted and transperineal template saturation biopsy.
Materials and methods: Data of all men who underwent prostate biopsy in our academic tertiary care center between 11/2016 and 10/2019 was prospectively collected. We developed a clinical prediction model for the detection of high-grade PCa (Gleason score ≥7) based on a multivariable logistic regression model incorporating age, PSA, prostate volume, digital rectal examination, family history, previous negative biopsy, 5-alpha-reductase inhibitor use and MRI PI-RADS score. BioPrev-C performance was externally validated in another prospective Swiss cohort and compared with two other PCa risk-calculators (SWOP-RC and PBCG-RC).
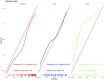
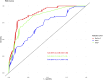
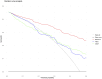
Results: Of 391 men in the development cohort, 157 (40.2%) were diagnosed with high-grade PCa. Validation of the BioPrev C revealed good discrimination with an area under the curve for high-grade PCa of 0.88 (95% Confidence Interval 0.82-0.93), which was higher compared to the other two risk calculators (0.71 for PBCG and 0.84 for SWOP). The BioPrev-C revealed good calibration in the low-risk range (0 - 0.25) and moderate overestimation in the intermediate risk range (0.25 - 0.75). The PBCG-RC showed good calibration and the SWOP-RC constant underestimation of high-grade PCa over the whole prediction range. Decision curve analyses revealed a clinical net benefit for the BioPrev-C at a clinical meaningful threshold probability range (≥4%), whereas PBCG and SWOP calculators only showed clinical net benefit above a 30% threshold probability.
Conclusion: BiopPrev-C is a novel contemporary risk calculator for the prediction of high-grade PCa. External validation of the BioPrev-C revealed relevant clinical benefit, which was superior compared to other well-known risk calculators. The BioPrev-C has the potential to significantly and safely reduce the number of men who should undergo a prostate biopsy.
Keywords: biopsy; decision aids; nomograms; prostate cancer; prostate-specific antigen.
Copyright © 2024 Hermanns, Wettstein, Kaufmann, Lautenbach, Kaufmann, Saba, Schmid, Hötker, Müntener, Umbehr and Poyet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Cavadas V, Osorio L, Sabell F, Teves F, Branco F, Silva-Ramos M. Prostate cancer prevention trial and European randomized study of screening for prostate cancer risk calculators: a performance comparison in a contemporary screened cohort. Eur Urol (2010) 58:551–8. doi: 10.1016/j.eururo.2010.06.023 - DOI - PubMed
-
- Van Poppel H, Roobol MJ, Chapple CR, Catto JWF, N’Dow J, Sonksen J, et al. Prostate-specific antigen testing as part of a risk-adapted early detection strategy for prostate cancer: European association of urology position and recommendations for 2021. Eur Urol (2021) 80:703–11. doi: 10.1016/j.eururo.2021.07.024 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

