Proceedings of the 11th Annual Deep Brain Stimulation Think Tank: pushing the forefront of neuromodulation with functional network mapping, biomarkers for adaptive DBS, bioethical dilemmas, AI-guided neuromodulation, and translational advancements
- PMID: 38450221
- PMCID: PMC10915873
- DOI: 10.3389/fnhum.2024.1320806
Proceedings of the 11th Annual Deep Brain Stimulation Think Tank: pushing the forefront of neuromodulation with functional network mapping, biomarkers for adaptive DBS, bioethical dilemmas, AI-guided neuromodulation, and translational advancements
Abstract
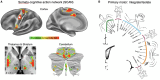
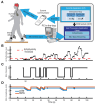
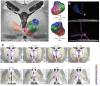
The Deep Brain Stimulation (DBS) Think Tank XI was held on August 9-11, 2023 in Gainesville, Florida with the theme of "Pushing the Forefront of Neuromodulation". The keynote speaker was Dr. Nico Dosenbach from Washington University in St. Louis, Missouri. He presented his research recently published in Nature inn a collaboration with Dr. Evan Gordon to identify and characterize the somato-cognitive action network (SCAN), which has redefined the motor homunculus and has led to new hypotheses about the integrative networks underpinning therapeutic DBS. The DBS Think Tank was founded in 2012 and provides an open platform where clinicians, engineers, and researchers (from industry and academia) can freely discuss current and emerging DBS technologies, as well as logistical and ethical issues facing the field. The group estimated that globally more than 263,000 DBS devices have been implanted for neurological and neuropsychiatric disorders. This year's meeting was focused on advances in the following areas: cutting-edge translational neuromodulation, cutting-edge physiology, advances in neuromodulation from Europe and Asia, neuroethical dilemmas, artificial intelligence and computational modeling, time scales in DBS for mood disorders, and advances in future neuromodulation devices.
Keywords: Parkinson's disease; adaptive DBS; artificial intelligence; deep brain stimulation (DBS); epilepsy; interventional psychiatry; neuroethics; optogenetics.
Copyright © 2024 Johnson, Dosenbach, Gordon, Welle, Wilkins, Bronte-Stewart, Voon, Morishita, Sakai, Merner, Lázaro-Muñoz, Williamson, Horn, Gilron, O'Keeffe, Gittis, Neumann, Little, Provenza, Sheth, Fasano, Holt-Becker, Raike, Moore, Pathak, Greene, Marceglia, Krinke, Tan, Bergman, Pötter-Nerger, Sun, Cabrera, McIntyre, Harel, Mayberg, Krystal, Pouratian, Starr, Foote, Okun and Wong.
Conflict of interest statement
ND has a financial interest in Turing Medical Inc. and may financially benefit if the company is successful in marketing FIRMM motion monitoring software products, may receive royalty income based on FIRMM technology developed at Washington University School of Medicine and Oregon Health and Sciences University and licensed to Turing Medical Inc, and is a co-founder of Turing Medical Inc. These potential conflicts of interest have been reviewed and are managed by Washington University School of Medicine. AH reports lecture fees for Boston Scientific and is a consultant for FxNeuromodulation and Abbott. RG is an employee of Rune Labs. JO'K is CEO and Founder of Machine Medicine Technologies. W-JN received honoraria for consulting from InBrain – Neuroelectronics that is a neurotechnology company and honoraria for talks from Medtronic that is a manufacturer of deep brain stimulation devices unrelated to this manuscript. SL has received speaking honoraria from Medtronic and is a consultant for Iota Biosciences. SS is a consultant for Zimmer Biomet, Neuropace, Koh Young, Boston Scientfic, Sensoria Therapeutics, Varian Medical; co-founder for Motif Neurotech. LM is an employee of Boston Scientific. AF has stock ownership in Inbrain Pharma and has received payments as consultant and/or speaker from Abbvie, Abbott, Boston Scientific, Ceregate, Dompé Farmaceutici, Inbrain Neuroelectronics, Ipsen, Medtronic, Iota, Syneos Health, Merz, Sunovion, Paladin Labs, UCB, Sunovion, and he has received research support from Abbvie, Boston Scientific, Medtronic, Praxis, ES and receives royalties from Springer. AH-B and RR are employees of Medtronic Inc. LM is an employee of Boston Scientific. YP is an employee of Abbott. DG is an employee of NeuroPace, owns NeuroPace stock, and has NeuroPace stock options. SM is a founder and shareholder of Newronika SpA. LK is the CEO of Newronika. MP-N has received speaker honoraria/travel costs from Medtronic, Boston Scientific, Abbott, Bial, and Abbvie and study reimbursements from Zambon, Licher, Boston Scientific, and Abbott. LC has received research support from National Institutes of Health and the National Network of Depression Centers and serves on the Board of Directors for the International Neuroethics Society (unpaid) as well as on the Advisory Council for the Institute of Neuroethics think and do thank (unpaid). CM is a paid consultant for Boston Scientific Neuromodulation, receives royalties from Hologram Consultants, Neuros Medical, Qr8 Health, and is a shareholder in the following companies: Hologram Consultants, Surgical Information Sciences, BrainDynamics, CereGate, Cardionomic, Enspire DBS. NH is a co-founder of Surgical Information Sciences (SIS), Inc. HM received consulting and IP licensing fees from Abbott Laboratories. AK has stock options in Neurawell and Big Health and is a consultant for Axsome Therapeutics, Big Health, Eisai, Evecxia, Harmony Biosciences, Idorsia, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Millenium Pharmaceuticals, Merck, Neurocrine Biosciences, Neurawell, Pernix, Otsuka Pharmaceuticals, Sage, and Takeda. NP is a consultant for Abbott and Sensoria Therapeutics. PS receives free research devices from Medtronic and fellowship support funding from Medtronic and BSCI and no personal income from anyone. KF reports receiving research support and fellowship support from Medtronic and Boston Scientific and research support from Functional Neuromodulation. MO serves as Medical Advisor the Parkinson's Foundation, and has received research grants from NIH, Parkinson's Foundation, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. MO has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford and Cambridge (movement disorders books). MO is an associate editor for New England Journal of Medicine Journal, Watch Neurology, and JAMA Neurology. MO has participated in CME and educational activities (past 12-24 months) on movement disorders sponsored by WebMD/Medscape, RMEI Medical Education, American Academy of Neurology, Movement Disorders Society, Mediflix and by Vanderbilt University. The institution and not MO receives grants from industry. MO has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Research projects at the University of Florida receive device and drug donations. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
Grants and funding
- P41 EB027061/EB/NIBIB NIH HHS/United States
- U01 NS119562/NS/NINDS NIH HHS/United States
- S10 OD025256/OD/NIH HHS/United States
- K23 NS120037/NS/NINDS NIH HHS/United States
- R01 NS090913/NS/NINDS NIH HHS/United States
- R01 MH130666/MH/NIMH NIH HHS/United States
- R01 MH133657/MH/NIMH NIH HHS/United States
- R01 MH096773/MH/NIMH NIH HHS/United States
- R25 NS108939/NS/NINDS NIH HHS/United States
- R44 MH121276/MH/NIMH NIH HHS/United States
- UH3 NS107709/NS/NINDS NIH HHS/United States
- R01 NS096008/NS/NINDS NIH HHS/United States
- K23 NS088590/NS/NINDS NIH HHS/United States
- R01 NS127892/NS/NINDS NIH HHS/United States
- RF1 MH117802/MH/NIMH NIH HHS/United States
- UH3 NS119844/NS/NINDS NIH HHS/United States
- R01 NS097782/NS/NINDS NIH HHS/United States
- UH3 NS103550/NS/NINDS NIH HHS/United States
- R01 MH113929/MH/NIMH NIH HHS/United States
- R01 NS113746/NS/NINDS NIH HHS/United States
- P50 NS123109/NS/NINDS NIH HHS/United States
- KL2 TR001429/TR/NCATS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 NS115975/NS/NINDS NIH HHS/United States
- R44 NS129521/NS/NINDS NIH HHS/United States
- UH3 NS103549/NS/NINDS NIH HHS/United States
- R01 NR014852/NR/NINR NIH HHS/United States
- R01 NS131405/NS/NINDS NIH HHS/United States
- R44 MH124567/MH/NIMH NIH HHS/United States
- R01 NS081118/NS/NINDS NIH HHS/United States
- R44 MH122066/MH/NIMH NIH HHS/United States
- UM1 NS132358/NS/NINDS NIH HHS/United States
- UG3 NS128150/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

