The human adenovirus PI3K-Akt activator E4orf1 is targeted by the tumor suppressor p53
- PMID: 38451084
- PMCID: PMC11019960
- DOI: 10.1128/jvi.01701-23
The human adenovirus PI3K-Akt activator E4orf1 is targeted by the tumor suppressor p53
Abstract
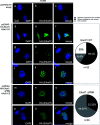
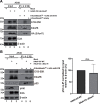
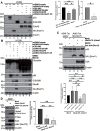
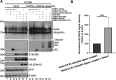
Human adenoviruses (HAdV) are classified as DNA tumor viruses due to their potential to mediate oncogenic transformation in non-permissive mammalian cells and certain human stem cells. To achieve transformation, the viral early proteins of the E1 and E4 regions must block apoptosis and activate proliferation: the former predominantly through modulating the cellular tumor suppressor p53 and the latter by activating cellular pro-survival and pro-metabolism protein cascades, such as the phosphoinositide 3-kinase (PI3K-Akt) pathway, which is activated by HAdV E4orf1. Focusing on HAdV-C5, we show that E4orf1 is necessary and sufficient to stimulate Akt activation through phosphorylation in H1299 cells, which is not only hindered but repressed during HAdV-C5 infection with a loss of E4orf1 function in p53-positive A549 cells. Contrary to other research, E4orf1 localized not only in the common, cytoplasmic PI3K-Akt-containing compartment, but also in distinct nuclear aggregates. We identified a novel inhibitory mechanism, where p53 selectively targeted E4orf1 to destabilize it, also stalling E4orf1-dependent Akt phosphorylation. Co-IP and immunofluorescence studies showed that p53 and E4orf1 interact, and since p53 is bound by the HAdV-C5 E3 ubiquitin ligase complex, we also identified E4orf1 as a novel factor interacting with E1B-55K and E4orf6 during infection; overexpression of E4orf1 led to less-efficient E3 ubiquitin ligase-mediated proteasomal degradation of p53. We hypothesize that p53 specifically subverts the pro-survival function of E4orf1-mediated PI3K-Akt activation to protect the cell from metabolic hyper-activation or even transformation.IMPORTANCEHuman adenoviruses (HAdV) are nearly ubiquitous pathogens comprising numerous subtypes that infect various tissues and organs. Among many encoded proteins that facilitate viral replication and subversion of host cellular processes, the viral E4orf1 protein has emerged as an intriguing yet under-investigated player in the complex interplay between the virus and its host. Nonetheless, E4orf1 has gained attention as a metabolism activator and oncogenic agent, while recent research is showing that E4orf1 may play a more important role in modulating the cellular pathways such as phosphoinositide 3-kinase-Akt-mTOR. Our study reveals a novel and general impact of E4orf1 on host mechanisms, providing a novel basis for innovative antiviral strategies in future therapeutic settings. Ongoing investigations of the cellular pathways modulated by HAdV are of great interest, particularly since adenovirus-based vectors actually serve as vaccine or gene vectors. HAdV constitute an ideal model system to analyze the underlying molecular principles of virus-induced tumorigenesis.
Keywords: E4orf1; HAdV; PI3K-Akt; p53; tumor suppressor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Rampersad S, Tennant P. 2018. Viruses, p 55–82
-
- Endter C, Dobner T. 2004. Edited by Doerfler W and Böhm P. Adenoviruses: model and vectors in virus-host interactions: immune system, oncogenesis, gene therapy, p 163–214. Springer, Berlin, Heidelberg.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

