Exploring factors shaping antibiotic resistance patterns in Streptococcus pneumoniae during the 2020 COVID-19 pandemic
- PMID: 38451256
- PMCID: PMC10923560
- DOI: 10.7554/eLife.85701
Exploring factors shaping antibiotic resistance patterns in Streptococcus pneumoniae during the 2020 COVID-19 pandemic
Abstract
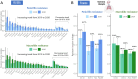
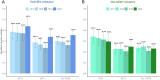
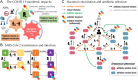
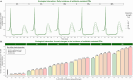
Non-pharmaceutical interventions implemented to block SARS-CoV-2 transmission in early 2020 led to global reductions in the incidence of invasive pneumococcal disease (IPD). By contrast, most European countries reported an increase in antibiotic resistance among invasive Streptococcus pneumoniae isolates from 2019 to 2020, while an increasing number of studies reported stable pneumococcal carriage prevalence over the same period. To disentangle the impacts of the COVID-19 pandemic on pneumococcal epidemiology in the community setting, we propose a mathematical model formalizing simultaneous transmission of SARS-CoV-2 and antibiotic-sensitive and -resistant strains of S. pneumoniae. To test hypotheses underlying these trends five mechanisms were built into the model and examined: (1) a population-wide reduction of antibiotic prescriptions in the community, (2) lockdown effect on pneumococcal transmission, (3) a reduced risk of developing an IPD due to the absence of common respiratory viruses, (4) community azithromycin use in COVID-19 infected individuals, (5) and a longer carriage duration of antibiotic-resistant pneumococcal strains. Among 31 possible pandemic scenarios involving mechanisms individually or in combination, model simulations surprisingly identified only two scenarios that reproduced the reported trends in the general population. They included factors (1), (3), and (4). These scenarios replicated a nearly 50% reduction in annual IPD, and an increase in antibiotic resistance from 20% to 22%, all while maintaining a relatively stable pneumococcal carriage. Exploring further, higher SARS-CoV-2 R0 values and synergistic within-host virus-bacteria interaction mechanisms could have additionally contributed to the observed antibiotic resistance increase. Our work demonstrates the utility of the mathematical modeling approach in unraveling the complex effects of the COVID-19 pandemic responses on AMR dynamics.
Keywords: COVID-19 pandemic; SARS-CoV-2; Streptococcus pneumoniae; antibiotic resistance; ecology; epidemiology; global health; invasive pneumococcal disease; virus-bacteria interactions.
© 2024, Kovacevic et al.
Conflict of interest statement
AK, DS, ER, SN, PH, EV, LT No competing interests declared, RC received consulting fees from Pfizer, Sanofi, MSD, and GSK, including travel grants from Pfizer and payments from Symposium Pfizer, MSD, GSK, and Sanofi, and participated in advisory/data safety monitoring board at Pfizer, Sanofi, MSD, and GSK, CL received travel grants from Pfizer and payments from Symposium Pfizer and MSD, LO received a research grant from Pfizer and Sanofi Pasteur on unrelated topics through her institution
Figures


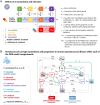
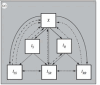
Update of
References
-
- Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, Wanjiru E, Jomo J, Musyimi R, Lipsitch M, Scott JAG. Rates of acquisition and clearance of pneumococcal serotypes in the nasopharynges of children in Kilifi District, Kenya. The Journal of Infectious Diseases. 2012;206:1020–1029. doi: 10.1093/infdis/jis447. - DOI - PMC - PubMed
-
- Alpkvist H, Athlin S, Nauclér P, Herrmann B, Abdeldaim G, Slotved HC, Hedlund J, Strålin K. Clinical and microbiological factors associated with high nasopharyngeal pneumococcal density in patients with pneumococcal pneumonia. PLOS ONE. 2015;10:e0140112. doi: 10.1371/journal.pone.0140112. - DOI - PMC - PubMed
-
- Amin-Chowdhury Z, Aiano F, Mensah A, Sheppard CL, Litt D, Fry NK, Andrews N, Ramsay ME, Ladhani SN. impact of the coronavirus disease 2019 (COVID-19) Pandemic on Invasive Pneumococcal Disease and Risk of Pneumococcal Coinfection With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Prospective National Cohort Study, England. Clinical Infectious Diseases. 2021;72:e65–e75. doi: 10.1093/cid/ciaa1728. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

