Phase I pharmacokinetic, safety, and preliminary efficacy study of tiragolumab in combination with atezolizumab in Chinese patients with advanced solid tumors
- PMID: 38451273
- PMCID: PMC11258083
- DOI: 10.1007/s00280-024-04650-y
Phase I pharmacokinetic, safety, and preliminary efficacy study of tiragolumab in combination with atezolizumab in Chinese patients with advanced solid tumors
Abstract
Purpose: Tiragolumab is an immunoglobulin G1 monoclonal antibody targeting the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor ITIM domains. Targeting multiple immune pathways may improve anti-tumor responses. The phase I YP42514 study assessed the pharmacokinetics (PK), safety, and preliminary efficacy of tiragolumab plus atezolizumab in Chinese patients with advanced solid tumors.
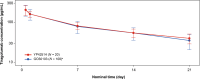
Methods: Adult patients from mainland China with Eastern Cooperative Oncology Group performance score 0/1, life expectancy of ≥ 12 weeks, and adequate hematologic/end organ function were eligible. Patients received tiragolumab 600 mg and atezolizumab 1200 mg intravenous every 3 weeks. Key endpoints were PK (serum concentrations of tiragolumab and atezolizumab) and safety. Results from this study were compared with the global phase I study, GO30103 (NCT02794571).
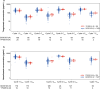
Results: In this study, 20 patients received a median of five doses of tiragolumab plus atezolizumab. Median age was 57.5 years, 85.0% of patients were male and the most common tumor type was non-small cell lung cancer. Exposures in Chinese patients were comparable to the global GO30103 population: geometric mean ratio was 1.07 for Cycle 1 tiragolumab area under the concentration-time curve0-21 and 0.92 and 0.93 for Cycle 1 peak and trough atezolizumab exposure, respectively. Treatment-related adverse events were consistent across the Chinese and global populations. Two patients (10.0%) in this study achieved a partial response.
Conclusion: In this study, tiragolumab plus atezolizumab was tolerable and demonstrated preliminary anti-tumor activity. There were no meaningful differences in the PK or safety of tiragolumab plus atezolizumab between the Chinese and global populations.
Clinical trial registration number: China Clinical Trial Registry Identifier CTR20210219/YP42514. Date of registration 16 March 2021.
Keywords: Advanced solid tumors; Combination anti-TIGIT and anti-PD-L1 cancer immunotherapy; Ethnic sensitivity and population bridging; Immune check point inhibitors; Phase I clinical trials.
© 2024. The Author(s).
Conflict of interest statement
C.S.S., H.D., P.C., Q.L., Y.C., and B.W. are employees of Genentech, Inc., and F. Hoffman La-Roche Ltd. stockholders. A.A. and Q.W. are employees of F. Hoffmann-La Roche Ltd. and F. Hoffman La-Roche Ltd, stockholders.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

