BMP4 and GREM1 are targets of SHH signaling and downstream regulators of collagen in the penis
- PMID: 38451311
- PMCID: PMC11063415
- DOI: 10.1093/jsxmed/qdae015
BMP4 and GREM1 are targets of SHH signaling and downstream regulators of collagen in the penis
Abstract
Background: Cavernous nerve (CN) injury, caused by prostatectomy and diabetes, initiates a remodeling process (smooth muscle apoptosis and increased collagen) in the corpora cavernosa of the penis of patients and animal models that is an underlying cause of erectile dysfunction (ED), and the Sonic hedgehog (SHH) pathway plays an essential role in the response of the penis to denervation, as collagen increases with SHH inhibition and decreases with SHH treatment.
Aim: We examined if part of the mechanism of how SHH prevents penile remodeling and increased collagen with CN injury involves bone morphogenetic protein 4 (BMP4) and gremlin1 (GREM1) and examined the relationship between SHH, BMP4, GREM1, and collagen in penis of ED patients and rat models of CN injury, SHH inhibition, and SHH, BMP4, and GREM1 treatment.
Methods: Corpora cavernosa of Peyronie's disease (control), prostatectomy, and diabetic ED patients were obtained (N = 30). Adult Sprague Dawley rats (n = 90) underwent (1) CN crush (1-7 days) or sham surgery; (2) CN injury and BMP4, GREM1, or mouse serum albumin (control) treatment via Affi-Gel beads or peptide amphiphile (PA) for 14 days; (3) 5E1 SHH inhibitor, IgG, or phosphate-buffered saline (control) treatment for 2 to 4 days; or (4) CN crush with mouse serum albumin or SHH for 9 days.
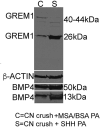
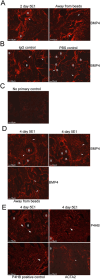
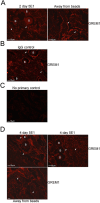
Outcomes: Immunohistochemical and Western analysis for BMP4 and GREM1, and collagen analysis by hydroxyproline and trichrome stain were performed.
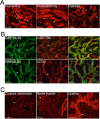
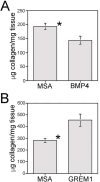
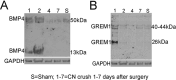
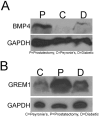
Results: BMP4 and GREM1 proteins were identified in corpora cavernosa smooth muscle of prostatectomy, diabetic, and Peyronie's patients, and in rat smooth muscle, sympathetic nerve fibers, perineurium, blood vessels, and urethra. Collagen decreased 25.4% in rats with CN injury and BMP4 treatment (P = .02) and increased 61.3% with CN injury and GREM1 treatment (P = .005). Trichrome stain showed increased collagen in rats treated with GREM1. Western analysis identified increased BMP4 and GREM1 in corpora cavernosa of prostatectomy and diabetic patients, and after CN injury (1-2 days) in our rat model. Localization of BMP4 and GREM1 changed with SHH inhibition. SHH treatment increased the monomer form of BMP4 and GREM1, altering their range of signaling.
Clinical implications: A better understanding of penile remodeling and how fibrosis occurs with loss of innervation is essential for development of novel ED therapies.
Strengths and limitations: The relationship between SHH, BMP4, GREM1, and collagen is complex in the penis.
Conclusion: BMP4 and GREM1 are downstream targets of SHH that impact collagen and may be useful in collaboration with SHH to prevent penile remodeling and ED.
Keywords: BMP4; GREM1; Peyronie’s; Sonic hedgehog; cavernous nerve injury; collagen; diabetes; erectile dysfunction.
© The Author(s) 2024. Published by Oxford University Press on behalf of The International Society of Sexual Medicine. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
SHH regulates penile morphology and smooth muscle through a mechanism involving BMP4 and GREM1.J Sex Med. 2024 Apr 30;21(5):379-390. doi: 10.1093/jsxmed/qdae016. J Sex Med. 2024. PMID: 38451321 Free PMC article.
-
Sonic hedgehog suppresses penile remodeling after cavernous nerve injury and sustains long-term normal penis morphology.J Sex Med. 2024 Oct 31;21(11):986-993. doi: 10.1093/jsxmed/qdae116. J Sex Med. 2024. PMID: 39279183
-
Novel control for erectile dysfunction research.J Sex Med. 2025 Aug 4;22(8):1322-1335. doi: 10.1093/jsxmed/qdaf135. J Sex Med. 2025. PMID: 40511860
-
Non-surgical therapies for Peyronie's disease.Cochrane Database Syst Rev. 2023 Jul 17;7(7):CD012206. doi: 10.1002/14651858.CD012206.pub2. Cochrane Database Syst Rev. 2023. PMID: 37490423 Free PMC article.
-
Sonic hedgehog, apoptosis, and the penis.J Sex Med. 2009 Mar;6 Suppl 3(Suppl 3):334-9. doi: 10.1111/j.1743-6109.2008.01192.x. J Sex Med. 2009. PMID: 19267857 Free PMC article. Review.
Cited by
-
Penile remodeling, Sonic hedgehog, and fibrosis.J Sex Med. 2025 May 10;22(5):668-670. doi: 10.1093/jsxmed/qdaf048. J Sex Med. 2025. PMID: 40356579 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

