SHH regulates penile morphology and smooth muscle through a mechanism involving BMP4 and GREM1
- PMID: 38451321
- PMCID: PMC11063416
- DOI: 10.1093/jsxmed/qdae016
SHH regulates penile morphology and smooth muscle through a mechanism involving BMP4 and GREM1
Abstract
Background: The cavernous nerve (CN) is frequently damaged in prostatectomy and diabetic patients with erectile dysfunction (ED), initiating changes in penile morphology including an acute and intense phase of apoptosis in penile smooth muscle and increased collagen, which alter penile architecture and make corpora cavernosa smooth muscle less able to relax in response to neurotransmitters, resulting in ED.
Aim: Sonic hedgehog (SHH) is a critical regulator of penile smooth muscle, and SHH treatment suppresses penile remodeling after CN injury through an unknown mechanism; we examine if part of the mechanism of how SHH preserves smooth muscle after CN injury involves bone morphogenetic protein 4 (BMP4) and gremlin1 (GREM1).
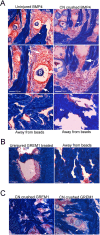
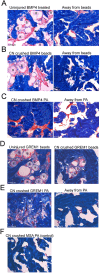
Methods: Primary cultures of smooth muscle cells were established from prostatectomy, diabetic, hypertension and Peyronie's (control) (N = 18) patients. Cultures were characterized by ACTA2, CD31, P4HB, and nNOS immunohistochemical analysis. Patient smooth muscle cell growth was quantified in response to BMP4 and GREM1 treatment. Adult Sprague Dawley rats underwent 1 of 3 surgeries: (1) uninjured or CN-injured rats were treated with BMP4, GREM1, or mouse serum albumin (control) proteins via Affi-Gel beads (N = 16) or peptide amphiphile (PA) (N = 26) for 3 and 14 days, and trichrome stain was performed; (2) rats underwent sham (N = 3), CN injury (N = 9), or CN injury and SHH PA treatment for 1, 2, and 4 days (N = 9).
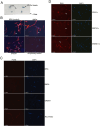
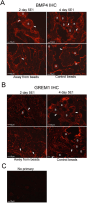
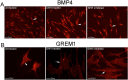
Outcomes: Western analysis for BMP4 and GREM1 was performed; (3) rats were treated with 5E1 SHH inhibitor (N = 6) or IgG (control; N = 6) for 2 and 4 days, and BMP4 and GREM1 localization was examined. Statistics were performed by analysis of variance with Scheffé's post hoc test.
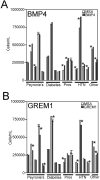
Results: BMP4 increased patient smooth muscle cell growth, and GREM1 decreased growth. In rats, BMP4 treatment via Affi-Gel beads and PA increased smooth muscle at 3 and 14 days of treatment. GREM1 treatment caused increased collagen and smooth muscle at 3 days, which switched to primarily collagen at 14 days. CN injury increased BMP4 and GREM1, while SHH PA altered Western band size, suggesting alternative cleavage and range of BMP4 and GREM1 signaling. SHH inhibition in rats increased BMP4 and GREM1 in fibroblasts.
Clinical implications: Understanding how SHH PA preserves and regenerates penile morphology after CN injury will aid development of ED therapies.
Strengths and limitations: SHH treatment alters BMP4 and GREM1 localization and range of signaling, which can affect penile morphology.
Conclusion: Part of the mechanism of how SHH regulates corpora cavernosa smooth muscle involves BMP4 and GREM1.
Keywords: BMP4; GREM1; Peyronie’s; Sonic hedgehog; cell culture; diabetes; erectile dysfunction; prostatectomy; smooth muscle.
© The Author(s) 2024. Published by Oxford University Press on behalf of The International Society of Sexual Medicine. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

