Stereotactic Radiation Therapy in Early Non-Small Cell Lung Cancer and Interstitial Lung Disease: A Nonrandomized Clinical Trial
- PMID: 38451491
- PMCID: PMC10921346
- DOI: 10.1001/jamaoncol.2023.7269
Stereotactic Radiation Therapy in Early Non-Small Cell Lung Cancer and Interstitial Lung Disease: A Nonrandomized Clinical Trial
Abstract
Importance: Patients with interstitial lung disease (ILD) and early-stage non-small cell lung cancer (NSCLC) have been reported to be at high risk of toxic effects after stereotactic ablative radiotherapy (SABR), but for many patients, there are limited alternative treatment options.
Objective: To prospectively assess the benefits and toxic effects of SABR in this patient population.
Design, setting, and participants: This prospective cohort study was conducted at 6 academic radiation oncology institutions, 5 in Canada and 1 in Scotland, with accrual between March 7, 2019, and January 12, 2022. Patients aged 18 years or older with fibrotic ILD and a diagnosis of T1-2N0 NSCLC who were not candidates for surgical resection were enrolled.
Intervention: Patients were treated with SABR to a dose of 50 Gy in 5 fractions every other day.
Main outcomes and measures: The study prespecified that SABR would be considered worthwhile if median overall survival-the primary end point-was longer than 1 year, with a grade 3 to 4 risk of toxic effects less than 35% and a grade 5 risk of toxic effects less than 15%. Secondary end points included toxic effects, progression-free survival (PFS), local control (LC), quality-of-life outcomes, and changes in pulmonary function. Intention-to-treat analysis was conducted.
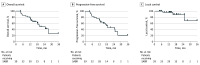
Results: Thirty-nine patients enrolled and received SABR. Median age was 78 (IQR, 67-83) years and 59% (n = 23) were male. At baseline, 70% (26 of 37) of patients reported dyspnea, median forced expiratory volume in first second of expiration was 80% (IQR, 66%-90%) predicted, median forced vital capacity was 84% (IQR, 69%-94%) predicted, and median diffusion capacity of the lung for carbon monoxide was 49% (IQR, 38%-61%) predicted. Median follow-up was 19 (IQR, 14-25) months. Overall survival at 1 year was 79% (95%, CI 62%-89%; P < .001 vs the unacceptable rate), and median overall survival was 25 months (95% CI, 14 months to not reached). Median PFS was 19 months (95% CI, 13-28 months), and 2-year LC was 92% (95% CI, 69%-98%). Adverse event rates (highest grade per patient) were grade 1 to 2: n = 12 (31%), grade 3: n = 4 (10%), grade 4: n = 0, and grade 5: n = 3 (7.7%, all due to respiratory deterioration).
Conclusions and relevance: In this trial, use of SABR in patients with fibrotic ILD met the prespecified acceptability thresholds for both toxicity and efficacy, supporting the use of SABR for curative-intent treatment after a careful discussion of risks and benefits.
Trial registration: ClinicalTrials.gov Identifier: NCT03485378.
Conflict of interest statement
Figures

Comment in
-
Prospective Data on Stereotactic Ablative Radiotherapy Provides Guidance in an Unusual Clinical Scenario.JAMA Oncol. 2024 May 1;10(5):582-583. doi: 10.1001/jamaoncol.2023.7039. JAMA Oncol. 2024. PMID: 38451539 No abstract available.
-
Navigating restriction from interstitial lung disease (ILD) with stereotactic ablative radiotherapy (SABR) in early-stage non-small cell lung cancer: soaring beyond the current treatment paradigm.Transl Cancer Res. 2025 Jan 31;14(1):11-15. doi: 10.21037/tcr-24-1813. Epub 2025 Jan 7. Transl Cancer Res. 2025. PMID: 39974416 Free PMC article. No abstract available.
References
-
- NCCN Clinical practice guidelines in oncology - non-small cell lung cancer version 1. 2023, December 22, 2022, https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
-
- Boily G, Filion É, Rakovich G, et al. ; Comité de l’évolution des pratiques en oncologie . Stereotactic ablative radiation therapy for the treatment of early-stage non–small-cell lung cancer: CEPO review and recommendations. J Thorac Oncol. 2015;10(6):872-882. doi:10.1097/JTO.0000000000000524 - DOI - PubMed
-
- Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. 2012;82(3):1149-1156. doi:10.1016/j.ijrobp.2011.03.005 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

