Water-Free Cyclosporine Ophthalmic Solution vs Vehicle for Dry Eye Disease: A Randomized Clinical Trial
- PMID: 38451509
- PMCID: PMC10921345
- DOI: 10.1001/jamaophthalmol.2024.0101
Water-Free Cyclosporine Ophthalmic Solution vs Vehicle for Dry Eye Disease: A Randomized Clinical Trial
Abstract
Importance: Dry eye disease (DED) is a prevalent eye disorder. Cyclosporine is an effective immunomodulator that is widely used in DED; however, due to its highly hydrophobic nature, delivery of cyclosporine to the ocular surface is challenging.
Objective: To evaluate the efficacy and safety of SHR8028, a water-free cyclosporine ophthalmic solution, 0.1%, compared with vehicle in Chinese participants with DED.
Design, setting, and participants: This was a multicenter, double-blind, vehicle-controlled, phase 3 randomized clinical trial conducted from March 4, 2021, to July 22, 2022. Adult participants with moderate to severe DED were recruited from 12 hospitals in China. Study data were analyzed April 2, 2022, for the primary analysis.
Interventions: Following a 14-day run-in period with an artificial tear, participants were randomly assigned (1:1) to receive water-free cyclosporine or vehicle (1 eye drop in each eye twice daily). After a 29-day treatment, participants of both groups were given the option to receive water-free cyclosporine for an additional 12 weeks for longer-term safety assessment.
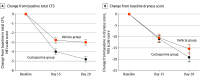
Main outcomes and measures: The primary end points were changes from baseline in total corneal fluorescein staining (tCFS) using the National Eye Institute scale and in dryness score on a visual analog scale at day 29.
Results: A total of 206 participants (mean [SD] age, 47.8 [14.2] years; 185 female [90%]) were enrolled, with 103 each in the cyclosporine group and the vehicle group. At day 29, the cyclosporine group experienced improved tCFS compared with vehicle (change [Δ] = -1.8; 95% CI, -2.7 to -1.0; P < .001), with a tCFS score decrease from baseline of -4.8 in the cyclosporine group and -3.0 in the vehicle group. Dryness score decreased from baseline in both groups (-19.2 vs -15.4; Δ = -3.8; 95% CI, -9.2 to 1.6; P = .17). During the 29-day treatment, treatment-related adverse events were reported in 15 participants (14.6%) in the cyclosporine group and 11 participants (10.7%) in the vehicle group.
Conclusions and relevance: Results demonstrated superiority of a water-free cyclosporine, 0.1%, eye solution over vehicle in improving tCFS score at day 29 in Chinese participants with DED. However, dryness score (VAS) was not improved at day 29.
Trial registration: ClinicalTrials.gov Identifier: NCT05841043.
Conflict of interest statement
Figures
Comment on
-
Unraveling the Potential of Water-Free Cyclosporine in Dry Eye Disease.JAMA Ophthalmol. 2024 Apr 1;142(4):343-344. doi: 10.1001/jamaophthalmol.2024.0224. JAMA Ophthalmol. 2024. PMID: 38451489 No abstract available.
References
-
- U.S. Food and Drug Administration . Restasis prescribing information. Accessed June 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/050790lbl.pdf
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

