Recent Advancements in Subcellular Proteomics: Growing Impact of Organellar Protein Niches on the Understanding of Cell Biology
- PMID: 38451675
- PMCID: PMC11296931
- DOI: 10.1021/acs.jproteome.3c00839
Recent Advancements in Subcellular Proteomics: Growing Impact of Organellar Protein Niches on the Understanding of Cell Biology
Abstract
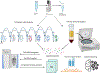
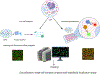
The mammalian cell is a complex entity, with membrane-bound and membrane-less organelles playing vital roles in regulating cellular homeostasis. Organellar protein niches drive discrete biological processes and cell functions, thus maintaining cell equilibrium. Cellular processes such as signaling, growth, proliferation, motility, and programmed cell death require dynamic protein movements between cell compartments. Aberrant protein localization is associated with a wide range of diseases. Therefore, analyzing the subcellular proteome of the cell can provide a comprehensive overview of cellular biology. With recent advancements in mass spectrometry, imaging technology, computational tools, and deep machine learning algorithms, studies pertaining to subcellular protein localization and their dynamic distributions are gaining momentum. These studies reveal changing interaction networks because of "moonlighting proteins" and serve as a discovery tool for disease network mechanisms. Consequently, this review aims to provide a comprehensive repository for recent advancements in subcellular proteomics subcontexting methods, challenges, and future perspectives for method developers. In summary, subcellular proteomics is crucial to the understanding of the fundamental cellular mechanisms and the associated diseases.
Keywords: APEX; BioID; computational tools; dynamic organellar maps; imaging technology; machine learning algorithms; mass spectrometry; proximity protein labeling; subcellular localization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Relationship between Pituitary Gland and Stem Cell in the Aspect of Hormone Production and Disease Prevention: A Narrative Review.Endocr Metab Immune Disord Drug Targets. 2025;25(7):509-526. doi: 10.2174/0118715303314551241031093717. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39812047 Review.
-
Single-cell proteomics analysis of human oocytes during GV-to-MI transition.Hum Reprod. 2025 Jul 1;40(7):1332-1343. doi: 10.1093/humrep/deaf086. Hum Reprod. 2025. PMID: 40359387
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
Cited by
-
Simultaneous profiling of native-state proteomes and transcriptomes of neural cell types using proximity labeling.bioRxiv [Preprint]. 2025 Feb 1:2025.01.29.635500. doi: 10.1101/2025.01.29.635500. bioRxiv. 2025. PMID: 39974879 Free PMC article. Preprint.
-
Integrating Proteomics into Personalized Medicine for Inflammatory Bowel Disease-Reality or Challenge?Int J Mol Sci. 2025 May 22;26(11):4993. doi: 10.3390/ijms26114993. Int J Mol Sci. 2025. PMID: 40507799 Free PMC article. Review.
References
-
- Regev A; Teichmann SA; Lander ES; Amit I; Benoist C; Birney E; Bodenmiller B; Campbell P; Carninci P; Clatworthy M; Clevers H; Deplancke B; Dunham I; Eberwine J; Eils R; Enard W; Farmer A; Fugger L; Göttgens B; Hacohen N; Haniffa M; Hemberg M; Kim S; Klenerman P; Kriegstein A; Lein E; Linnarsson S; Lundberg E; Lundeberg J; Majumder P; Marioni JC; Merad M; Mhlanga M; Nawijn M; Netea M; Nolan G; Pe’er D; Phillipakis A; Ponting CP; Quake S; Reik W; Rozenblatt-Rosen O; Sanes J; Satija R; Schumacher TN; Shalek A; Shapiro E; Sharma P; Shin JW; Stegle O; Stratton M; Stubbington MJT; Theis FJ; Uhlen M; van Oudenaarden A; Wagner A; Watt F; Weissman J; Wold B; Xavier R; Yosef N Human Cell Atlas Meeting Participants. The Human Cell Atlas. Elife 2017, 6, No. e27041. - PMC - PubMed
-
- Jimenez CR; Verheul HM W. Mass Spectrometry-Based Proteomics: From Cancer Biology to Protein Biomarkers, Drug Targets, and Clinical Applications. Am. Soc. Clin Oncol Educ Book 2014, 34, No. e504. - PubMed
-
- Hosp F; Mann M A Primer on Concepts and Applications of Proteomics in Neuroscience. Neuron 2017, 96 (3), 558–571. - PubMed
-
- Thul PJ; Åkesson L; Wiking M; Mahdessian D; Geladaki A; Ait Blal H; Alm T; Asplund A; Björk L; Breckels LM; Bäckström A; Danielsson F; Fagerberg L; Fall J; Gatto L; Gnann C; Hober S; Hjelmare M; Johansson F; Lee S; Lindskog C; Mulder J; Mulvey CM; Nilsson P; Oksvold P; Rockberg J; Schutten R; Schwenk JM; Sivertsson C5; Sjöstedt E; Skogs M; Stadler C; Sullivan DP; Tegel H; Winsnes C; Zhang C; Zwahlen M; Mardinoglu A; Pontén F; von Feilitzen K; Lilley KS; Uhlén M; Lundberg E A Subcellular Map of the Human Proteome. Science 2017, 356 (6340), No. eaal3321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

