An in vivo screening platform identifies senolytic compounds that target p16INK4a+ fibroblasts in lung fibrosis
- PMID: 38451724
- PMCID: PMC11060735
- DOI: 10.1172/JCI173371
An in vivo screening platform identifies senolytic compounds that target p16INK4a+ fibroblasts in lung fibrosis
Abstract
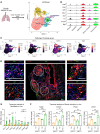
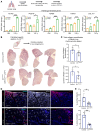
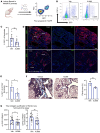
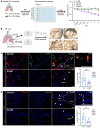
The appearance of senescent cells in age-related diseases has spurred the search for compounds that can target senescent cells in tissues, termed senolytics. However, a major caveat with current senolytic screens is the use of cell lines as targets where senescence is induced in vitro, which does not necessarily reflect the identity and function of pathogenic senescent cells in vivo. Here, we developed a new pipeline leveraging a fluorescent murine reporter that allows for isolation and quantification of p16Ink4a+ cells in diseased tissues. By high-throughput screening in vitro, precision-cut lung slice (PCLS) screening ex vivo, and phenotypic screening in vivo, we identified a HSP90 inhibitor, XL888, as a potent senolytic in tissue fibrosis. XL888 treatment eliminated pathogenic p16Ink4a+ fibroblasts in a murine model of lung fibrosis and reduced fibrotic burden. Finally, XL888 preferentially targeted p16INK4a-hi human lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis (IPF), and reduced p16INK4a+ fibroblasts from IPF PCLS ex vivo. This study provides proof of concept for a platform where p16INK4a+ cells are directly isolated from diseased tissues to identify compounds with in vivo and ex vivo efficacy in mice and humans, respectively, and provides a senolytic screening platform for other age-related diseases.
Keywords: Aging; Cellular senescence; Drug screens; Fibrosis; Pulmonology.
Conflict of interest statement
Figures



Comment in
- A pipeline for senolytics
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

