DNA topoisomerase II inhibition potentiates osimertinib's therapeutic efficacy in EGFR-mutant non-small cell lung cancer models
- PMID: 38451729
- PMCID: PMC11093598
- DOI: 10.1172/JCI172716
DNA topoisomerase II inhibition potentiates osimertinib's therapeutic efficacy in EGFR-mutant non-small cell lung cancer models
Abstract
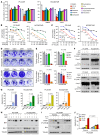
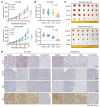
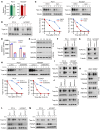
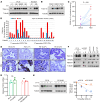
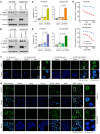
Development of effective strategies to manage the inevitable acquired resistance to osimertinib, a third-generation EGFR inhibitor for the treatment of EGFR-mutant (EGFRm) non-small cell lung cancer (NSCLC), is urgently needed. This study reports that DNA topoisomerase II (Topo II) inhibitors, doxorubicin and etoposide, synergistically decreased cell survival, with enhanced induction of DNA damage and apoptosis in osimertinib-resistant cells; suppressed the growth of osimertinib-resistant tumors; and delayed the emergence of osimertinib-acquired resistance. Mechanistically, osimertinib decreased Topo IIα levels in EGFRm NSCLC cells by facilitating FBXW7-mediated proteasomal degradation, resulting in induction of DNA damage; these effects were lost in osimertinib-resistant cell lines that possess elevated levels of Topo IIα. Increased Topo IIα levels were also detected in the majority of tissue samples from patients with NSCLC after relapse from EGFR tyrosine kinase inhibitor treatment. Enforced expression of an ectopic TOP2A gene in sensitive EGFRm NSCLC cells conferred resistance to osimertinib, whereas knockdown of TOP2A in osimertinib-resistant cell lines restored their susceptibility to osimertinib-induced DNA damage and apoptosis. Together, these results reveal an essential role of Topo IIα inhibition in mediating the therapeutic efficacy of osimertinib against EGFRm NSCLC, providing scientific rationale for targeting Topo II to manage acquired resistance to osimertinib.
Keywords: Apoptosis; Lung cancer; Oncology; Telomeres; Therapeutics.
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

