ALK upregulates POSTN and WNT signaling to drive neuroblastoma
- PMID: 38451815
- PMCID: PMC11101011
- DOI: 10.1016/j.celrep.2024.113927
ALK upregulates POSTN and WNT signaling to drive neuroblastoma
Abstract
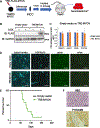
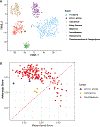
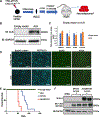
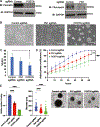
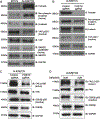
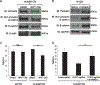
Neuroblastoma is the most common extracranial solid tumor of childhood. While MYCN and mutant anaplastic lymphoma kinase (ALKF1174L) cooperate in tumorigenesis, how ALK contributes to tumor formation remains unclear. Here, we used a human stem cell-based model of neuroblastoma. Mis-expression of ALKF1174L and MYCN resulted in shorter latency compared to MYCN alone. MYCN tumors resembled adrenergic, while ALK/MYCN tumors resembled mesenchymal, neuroblastoma. Transcriptomic analysis revealed enrichment in focal adhesion signaling, particularly the extracellular matrix genes POSTN and FN1 in ALK/MYCN tumors. Patients with ALK-mutant tumors similarly demonstrated elevated levels of POSTN and FN1. Knockdown of POSTN, but not FN1, delayed adhesion and suppressed proliferation of ALK/MYCN tumors. Furthermore, loss of POSTN reduced ALK-dependent activation of WNT signaling. Reciprocally, inhibition of the WNT pathway reduced expression of POSTN and growth of ALK/MYCN tumor cells. Thus, ALK drives neuroblastoma in part through a feedforward loop between POSTN and WNT signaling.
Keywords: ALK; CP: Cancer; MYCN; POSTN; WNT; human pluripotent stem cells; neuroblastoma.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests W.C.G. and N.N. are employees and shareholders at Revolution Medicines (Redwood City, CA, USA). W.A.W. is a co-founder of StemSynergy Therapeutics.
Figures

References
-
- van Groningen T, Akogul N, Westerhout EM, Chan A, Hasselt NE, Zwijnenburg DA, Broekmans M, Stroeken P, Haneveld F, Hooijer GKJ, et al.,. A NOTCH feed-forward loop drives reprogramming from adrenergic to mesenchymal state in neuroblastoma. Nat. Commun.. 04 April 2019;10:1530. doi:10.1038/s41467-019-09470-w - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

