Hepatic Snai1 and Snai2 promote liver regeneration and suppress liver fibrosis in mice
- PMID: 38451818
- PMCID: PMC11025633
- DOI: 10.1016/j.celrep.2024.113875
Hepatic Snai1 and Snai2 promote liver regeneration and suppress liver fibrosis in mice
Abstract
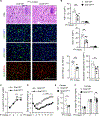
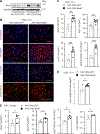
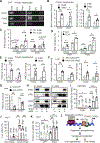
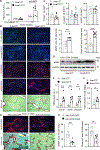
Liver injury stimulates hepatocyte replication and hepatic stellate cell (HSC) activation, thereby driving liver regeneration. Aberrant HSC activation induces liver fibrosis. However, mechanisms underlying liver regeneration and fibrosis remain poorly understood. Here, we identify hepatic Snai1 and Snai2 as important transcriptional regulators for liver regeneration and fibrosis. Partial hepatectomy or CCl4 treatment increases occupancies of Snai1 and Snai2 on cyclin A2 and D1 promoters in the liver. Snai1 and Snai2 in turn increase promoter H3K27 acetylation and cyclin A2/D1 expressions. Hepatocyte-specific deletion of both Snai1 and Snai2, but not one alone, suppresses liver cyclin A2/D1 expression and regenerative hepatocyte proliferation after hepatectomy or CCl4 treatments but augments CCl4-stimulated HSC activation and liver fibrosis. Conversely, Snai2 overexpression in the liver enhances hepatocyte replication and suppresses liver fibrosis after CCl4 treatment. These results suggest that hepatic Snai1 and Snai2 directly promote, via histone modifications, reparative hepatocyte replication and indirectly inhibit liver fibrosis.
Keywords: CCl4; CP: Cell biology; CP: Developmental biology; Snai1; Snai2; hepatectomy; hepatocyte replication; histone acetylation; histone modifications; liver fibrosis; liver regeneration.
Copyright © 2024 University of Michigan. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Constitutive Activation of the Nlrc4 Inflammasome Prevents Hepatic Fibrosis and Promotes Hepatic Regeneration after Partial Hepatectomy.Mediators Inflamm. 2015;2015:909827. doi: 10.1155/2015/909827. Epub 2015 Nov 9. Mediators Inflamm. 2015. PMID: 26635450 Free PMC article.
-
MicroRNA-203 promotes liver regeneration after partial hepatectomy in cirrhotic rats.J Surg Res. 2017 May 1;211:53-63. doi: 10.1016/j.jss.2016.11.043. Epub 2016 Nov 28. J Surg Res. 2017. PMID: 28501131
-
Partial hepatectomy-induced regeneration accelerates reversion of liver fibrosis involving participation of hepatic stellate cells.Exp Biol Med (Maywood). 2008 Jul;233(7):827-39. doi: 10.3181/0709-RM-247. Epub 2008 Apr 29. Exp Biol Med (Maywood). 2008. PMID: 18445764
-
MicroRNA-30a Suppresses the Activation of Hepatic Stellate Cells by Inhibiting Epithelial-to-Mesenchymal Transition.Cell Physiol Biochem. 2018;46(1):82-92. doi: 10.1159/000488411. Epub 2018 Mar 20. Cell Physiol Biochem. 2018. PMID: 29587268
-
Growth factors in liver development, regeneration and carcinogenesis.Prog Growth Factor Res. 1991;3(3):219-34. doi: 10.1016/0955-2235(91)90008-r. Prog Growth Factor Res. 1991. PMID: 1667366 Review.
Cited by
-
Advances in intrahepatic and extrahepatic vascular dysregulations in cirrhotic portal hypertension.Front Med (Lausanne). 2025 Jan 31;12:1515400. doi: 10.3389/fmed.2025.1515400. eCollection 2025. Front Med (Lausanne). 2025. PMID: 39958826 Free PMC article. Review.
-
Molecular mechanisms in liver repair and regeneration: from physiology to therapeutics.Signal Transduct Target Ther. 2025 Feb 8;10(1):63. doi: 10.1038/s41392-024-02104-8. Signal Transduct Target Ther. 2025. PMID: 39920130 Free PMC article. Review.
-
Sexually Dimorphic Effects of CYP2B6 in the Development of Fasting-Mediated Steatosis in Mice: Role of the Oxylipin Products 9-HODE and 9-HOTrE.Biomedicines. 2025 Jan 25;13(2):295. doi: 10.3390/biomedicines13020295. Biomedicines. 2025. PMID: 40002708 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

