Transition Path Sampling Study of Engineered Enzymes That Catalyze the Morita-Baylis-Hillman Reaction: Why Is Enzyme Design so Difficult?
- PMID: 38451822
- PMCID: PMC10963169
- DOI: 10.1021/acs.jcim.4c00045
Transition Path Sampling Study of Engineered Enzymes That Catalyze the Morita-Baylis-Hillman Reaction: Why Is Enzyme Design so Difficult?
Abstract
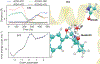
It is hoped that artificial enzymes designed in laboratories can be efficient alternatives to chemical catalysts that have been used to synthesize organic molecules. However, the design of artificial enzymes is challenging and requires a detailed molecular-level analysis to understand the mechanism they promote in order to design efficient variants. In this study, we computationally investigate the mechanism of proficient Morita-Baylis-Hillman enzymes developed using a combination of computational design and directed evolution. The powerful transition path sampling method coupled with in-depth post-processing analysis has been successfully used to elucidate the different chemical pathways, transition states, protein dynamics, and free energy barriers of reactions catalyzed by such laboratory-optimized enzymes. This research provides an explanation for how different chemical modifications in an enzyme affect its catalytic activity in ways that are not predictable by static design algorithms.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





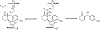
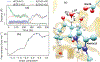

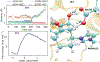

Similar articles
-
Directed Evolution's Selective Use of Quantum Tunneling in Designed Enzymes─A Combined Theoretical and Experimental Study.J Phys Chem B. 2025 Feb 6;129(5):1555-1562. doi: 10.1021/acs.jpcb.4c08169. Epub 2025 Jan 28. J Phys Chem B. 2025. PMID: 39874479
-
Engineering an efficient and enantioselective enzyme for the Morita-Baylis-Hillman reaction.Nat Chem. 2022 Mar;14(3):313-320. doi: 10.1038/s41557-021-00833-9. Epub 2021 Dec 16. Nat Chem. 2022. PMID: 34916595 Free PMC article.
-
Enantioselective, organocatalytic Morita-Baylis-Hillman and Aza-Morita-Baylis-Hillman reactions: stereochemical issues.Molecules. 2010 Feb 1;15(2):709-34. doi: 10.3390/molecules15020709. Molecules. 2010. PMID: 20335941 Free PMC article. Review.
-
Multifunctional chiral phosphine organocatalysts in catalytic asymmetric Morita-Baylis-Hillman and related reactions.Acc Chem Res. 2010 Jul 20;43(7):1005-18. doi: 10.1021/ar900271g. Acc Chem Res. 2010. PMID: 20232829
-
Biological Activities of Morita-Baylis-Hillman Adducts (MBHA).Mini Rev Med Chem. 2023;23(17):1691-1710. doi: 10.2174/1389557523666230202103719. Mini Rev Med Chem. 2023. PMID: 36733204 Review.
Cited by
-
Targeted TPS Shooting Using Computer Vision to Generate Ensemble of Trajectories.J Chem Theory Comput. 2025 Apr 8;21(7):3353-3359. doi: 10.1021/acs.jctc.4c01725. Epub 2025 Mar 17. J Chem Theory Comput. 2025. PMID: 40098324
-
Transition Path Sampling Based Free Energy Calculations of Evolution's Effect on Rates in β-Lactamase: The Contributions of Rapid Protein Dynamics to Rate.J Phys Chem B. 2024 Nov 28;128(47):11658-11665. doi: 10.1021/acs.jpcb.4c06689. Epub 2024 Nov 13. J Phys Chem B. 2024. PMID: 39536181
-
The Evolution of the Acylation Mechanism in β-Lactamase and Rapid Protein Dynamics.ACS Catal. 2024 Sep 20;14(18):13640-13651. doi: 10.1021/acscatal.4c03065. Epub 2024 Aug 28. ACS Catal. 2024. PMID: 39464311
-
Directed Evolution's Selective Use of Quantum Tunneling in Designed Enzymes─A Combined Theoretical and Experimental Study.J Phys Chem B. 2025 Feb 6;129(5):1555-1562. doi: 10.1021/acs.jpcb.4c08169. Epub 2025 Jan 28. J Phys Chem B. 2025. PMID: 39874479
References
-
- Wolfenden R; Snider MJ The Depth of Chemical Time and the Power of Enzymes as Catalysts. Acc. Chem. Res 2001, 34, 938–945. - PubMed
-
- Bar-Even A; Noor E; Savir Y; Liebermeister W; Davidi D; Tawfik DS; Milo R The Moderately Efficient Enzyme: Evolutionary and Physicochemical Trends Shaping Enzyme Parameters. Biochem 2011, 50, 4402–4410. - PubMed
-
- Schramm VL Enzymatic transition states and transition state analog design. Annu. Rev. Biochem 1998, 67, 693–720. - PubMed
-
- Kuah E; Toh S; Yee J; Ma Q; Gao Z Enzyme Mimics: Advances and Applications. Chem. - Eur. J 2016, 22, 8404–8430. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

