Oviduct and endometrial epithelium improve in vitro produced bovine embryo developmental kinetics
- PMID: 38451876
- PMCID: PMC11056959
- DOI: 10.1530/REP-24-0008
Oviduct and endometrial epithelium improve in vitro produced bovine embryo developmental kinetics
Abstract
In brief: Standard in vitro produced (IVP) bovine embryo culture media limit embryonic development. Culturing IVP bovine embryos in standard IVP bovine embryo culture media conditioned with oviduct and/or endometrial cells improves blastocyst formation and reduces the time to formation.
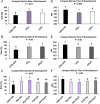
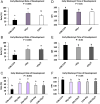
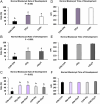
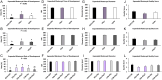
Abstract: In vitro embryo production in cattle greatly impacts blastomere biochemistry, embryo rate of development and pre- and post-transfer survival. In vivo, the bovine embryo migrates through the oviduct isthmus before entering the uterus on approximately day 4 of development where it remains unattached within the uterine lumen until day 20 of gestation. During this time, the embryo is sequentially exposed to oviduct followed by endometrial secretions that support embryonic development. Considering this, we tested the effect of culturing in vitro produced (IVP) bovine embryos sequentially in oviduct epithelial- (OEp; days 1-3) followed by endometrial epithelial- (EEp) or EEp and fibroblast cell (EEp/F; days 4-8)-conditioned media on embryonic development using a time-lapse monitoring system. Compared to control, culturing IVP embryos in EEp- or EEp/F-conditioned media without prior culture in OEp-conditioned media increased blastocyst formation (P < 0.05) and reduced the time to blastocyst formation (P < 0.05). Culturing IVP bovine embryos in OEp-conditioned media followed by EEp- or EEp/F-conditioned media, however, had the greatest impact on embryo developmental kinetics and increased morula and blastocyst formation (P < 0.05) and reduced time to formation (P < 0.05). Day 8 blastocyst cell numbers, diameter and quality were not significantly different, although, blastocyst quality scores were less (indicative of better quality) for all cell-conditioned media compared to control. In conclusion, IVP bovine embryo development may be improved using a sequential embryo culture system involving bovine oviduct followed by endometrial cell-conditioned media.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Figures



References
-
- Biase FH, Rabel C, Guillomot M, Hue I, Andropolis K, Olmstead CA, Oliveira R, Wallace R, Le Bourhis D, Richard C, et al.2016Massive dysregulation of genes involved in cell signaling and placental development in cloned cattle conceptus and maternal endometrium. PNAS 11314492–14501. ( 10.1073/pnas.1520945114) - DOI - PMC - PubMed
-
- Bó GA & Mapletoft RJ. 2013Evaluation and classification of bovine embryos. Animal Reproduction 10344–348.

