Clinical outcomes and prognostic factors after HCV clearance with DAA in HIV/HCV-coinfected patients with advanced fibrosis/cirrhosis
- PMID: 38452004
- PMCID: PMC11643110
- DOI: 10.1097/HEP.0000000000000838
Clinical outcomes and prognostic factors after HCV clearance with DAA in HIV/HCV-coinfected patients with advanced fibrosis/cirrhosis
Abstract
Background and aims: We assessed long-term clinical outcomes and prognostic factors for liver disease progression after sustained viral response with direct-acting antivirals in patients coinfected with HIV/HCV with advanced fibrosis or cirrhosis.
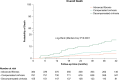
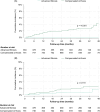
Approach and results: A total of 1300 patients who achieved sustained viral response with direct-acting antivirals from 2014 to 2017 in Spain were included: 1145 with compensated advanced chronic liver disease (384 advanced fibrosis and 761 compensated cirrhosis) and 155 with decompensated cirrhosis. The median follow-up was 40.9 months. Overall, 85 deaths occurred, 61 due to non-liver non-AIDS-related causes that were the leading cause of death across all stages of liver disease. The incidence (95% CI) of decompensation per 100 person-years (py) was 0 in patients with advanced fibrosis, 1.01 (0.68-1.51) in patients with compensated cirrhosis, and 8.35 (6.05-11.53) in patients with decompensated cirrhosis. The incidence (95% CI) of HCC per 100 py was 0.34 (0.13-0.91) in patients with advanced fibrosis, 0.73 (0.45-1.18) in patients with compensated cirrhosis, and 1.92 (1.00-3.70) per 100 py in patients with decompensated cirrhosis. Prognostic factors for decompensation in patients with compensated advanced chronic liver disease included serum albumin, liver stiffness measurement (LSM), and fibrosis 4. In this population, LSM and LSM-based posttreatment risk stratification models showed their predictive ability for decompensation and HCC.
Conclusions: Non-liver non-AIDS-related events were the leading causes of morbidity and mortality after direct-acting antiviral cure among coinfected patients with advanced fibrosis/cirrhosis. Among those with compensated advanced chronic liver disease, baseline LSM and posttreatment LSM-based models helped to assess decompensation and HCC risk.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Juan Berenguer advises, is on the speakers’ bureau, and received grants from Gilead, MSD, and ViiV Healthcare. He advises and is on the speakers’ bureau for AbbVie, GlaxoSmithKline, and Janssen. Teresa Aldámiz-Echevarría advises and is on the speakers’ bureau for Gilead, Janssen, MSD, and ViiV Healthcare. Chiara Fanciulli is employed by Gilead, Janssen, MSD, and ViiV Healthcare. Jorge Vergas advises and is on the speakers’ bureau for Janssen and ViiV Healthcare. He is on the speakers’ bureau for Gilead. Rafael Bañares advises and is on the speakers’ bureau for AbbVie, Gore, and Gilead. Juan González-García advises, is on the speakers’ bureau, and received grants from Gilead, MSD, and ViiV Healthcare. He advises and is on the speakers’ bureau for Janssen. The remaining authors have no conflicts to report.
Figures

References
-
- Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet. 2019;393:1453–1464. - PubMed
-
- Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2019;69:487–497. - PubMed
-
- Krassenburg LAP, Maan R, Ramji A, Manns MP, Cornberg M, Wedemeyer H, et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J Hepatol. 2021;74:1053–1063. - PubMed
-
- Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–747. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

