Fitusiran prophylaxis in people with hemophilia A or B who switched from prior BPA/CFC prophylaxis: the ATLAS-PPX trial
- PMID: 38452197
- PMCID: PMC11181353
- DOI: 10.1182/blood.2023021864
Fitusiran prophylaxis in people with hemophilia A or B who switched from prior BPA/CFC prophylaxis: the ATLAS-PPX trial
Abstract
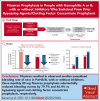
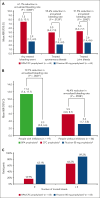
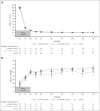
Fitusiran, a subcutaneous investigational small interfering RNA therapeutic, targets antithrombin to rebalance hemostasis in people with hemophilia A or B (PwHA/B), irrespective of inhibitor status. This phase 3, open-label study evaluated the efficacy and safety of fitusiran prophylaxis in males aged ≥12 years with hemophilia A or B, with or without inhibitors, who received prior bypassing agent (BPA)/clotting factor concentrate (CFC) prophylaxis. Participants continued their prior BPA/CFC prophylaxis for 6 months before switching to once-monthly 80 mg fitusiran prophylaxis for 7 months (onset and efficacy periods). Primary end point was annualized bleeding rate (ABR) in the BPA/CFC prophylaxis and fitusiran efficacy period. Secondary end points included spontaneous ABR (AsBR) and joint ABR (AjBR). Safety and tolerability were assessed. Of 80 enrolled participants, 65 (inhibitor, n = 19; noninhibitor, n = 46) were eligible for ABR analyses. Observed median ABRs were 6.5 (interquartile range [IQR], 2.2-19.6)/4.4 (IQR, 2.2-8.7) with BPA/CFC prophylaxis vs 0.0 (IQR, 0.0-0.0)/0.0 (IQR, 0.0-2.7) in the corresponding fitusiran efficacy period. Estimated mean ABRs were substantially reduced with fitusiran by 79.7% (P = .0021) and 46.4% (P = .0598) vs BPA/CFC prophylaxis, respectively. Forty-one participants (63.1%) experienced 0 treated bleeds with fitusiran vs 11 (16.9%) with BPAs/CFCs. Median AsBR and AjBR were both 2.2 with BPA/CFC prophylaxis and 0.0 in the fitusiran efficacy period. Two participants (3.0%) experienced suspected or confirmed thromboembolic events with fitusiran. Once-monthly fitusiran prophylaxis significantly reduced bleeding events vs BPA/CFC prophylaxis in PwHA/B, with or without inhibitors, and reported adverse events were generally consistent with previously identified risks of fitusiran. This trial was registered at www.ClinicalTrials.gov as #NCT03549871.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: B.N. has been a study investigator for Sobi, Biogen/Bioverativ, CSL, Bayer, and Sanofi; and received honoraria from Sobi (honoraria donated to the Irish Haemophilia Society). B.Z. has acted in advisory board and/or provided consultancy for Pfizer, Shire, Novo Nordisk, Roche, Sobi, Bayer, and BioMarin. B.A. has received honoraria and has acted in advisory board and/or provided consultancy for Pfizer, Takeda, Novo Nordisk, Roche, Sobi, Bayer, and BioMarin Pharmaceutical. P.K. has received honoraria as a member of an advisory board and/or speaker from BioMarin, Novo Nordisk, Roche, Takeda, AbbVie, and CSL Behring. T.M. has received honoraria from Bayer, Takeda/Shire, Novo Nordisk, Bioverative/Sanofi, CSL Behring, and Chugai. F.P. has received honoraria from Roche, Sanofi, Sobi, and Takeda; and is a member of advisory boards of Roche, Sanofi, Sobi, and Takeda. G.K. consults for Alnylam, Bayer, BioMarin Pharmaceutical, CSL, Novo Nordisk, Opko Biologics, Pfizer, Takeda, Roche, Sanofi, and uniQure; receives research funding from Alnylam, Bayer, BPL, Opko Biologics, Pfizer, Roche, and Takeda; has been involved with speaker bureaus for Bayer, Pfizer, CSL, Shire, Novo Nordisk, and Roche; and has a membership on an entity’s board of directors or advisory committees for Alnylam, Bayer, BioMarin Pharmaceutical, CSL, Novo Nordisk, Opko Biologics, Pfizer, Takeda, Roche, Sanofi, and uniQure. C.N. received honoraria/consultancy fees from BioMarin, Novo Nordisk, Roche, Sanofi, Sobi, Takeda, Pfizer, Spark, and Bayer; grants from Novo Nordisk, Roche, Sanofi, and Sobi; and has a membership on an entity’s board of directors or advisory committees for BioMarin, Novo Nordisk, Roche, Sanofi, Takeda, Pfizer, Spark, Bayer, CSL Behring, and uniQure. G.Y. has received grants from Genentech/Roche, Grifols, and Takeda; and speaking and consultancy fees from ApcinteX, BioMarin, CSL Behring, Genentech/Roche, Grifols, Hema Biologics/LFB, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda. J.C. and B.K. are current employees of Sanofi. C.S. is a current employee and stockholder in Sanofi. F.S. is a current employee in Sanofi and equity holder in Sanofi. S.A. is an employee and equity holder in Sanofi; and a member of the WEST advisory committee. B.M. was an employee and equity holder in Sanofi at the time of the study; also has divested equity in Sanofi in the past 24 months; and is an employee of Editas Medicine. K.K. received honoraria from Pfizer, Bayer, Takeda, Roche, and Novo Nordisk; has attended speaker bureaus for Pfizer, Bayer, Takeda, Roche, and Novo Nordisk; and has a membership on an entity's board of directors or advisory committees for Pfizer, Bayer, Takeda, Roche, and Novo Nordisk. The remaining authors declare no competing financial interests.
Figures

Comment in
-
RNAing toward a new therapy for hemophilia.Blood. 2024 May 30;143(22):2219-2221. doi: 10.1182/blood.2024024295. Blood. 2024. PMID: 38814651 No abstract available.
References
-
- Nogami K, Shima M. New therapies using nonfactor products for patients with hemophilia and inhibitors. Blood. 2019;133(5):399–406. - PubMed
-
- Negrier C, Shima M, Hoffman M. The central role of thrombin in bleeding disorders. Blood Rev. 2019;38 - PubMed
-
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1–158. - PubMed
-
- Willyard C. Thrombosis: balancing act. Nature. 2014;515(7528):S168–169. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

