N-Cα Bond Cleavage Catalyzed by a Multinuclear Iron Oxygenase from a Divergent Methanobactin-like RiPP Gene Cluster
- PMID: 38452252
- PMCID: PMC11062405
- DOI: 10.1021/jacs.3c11740
N-Cα Bond Cleavage Catalyzed by a Multinuclear Iron Oxygenase from a Divergent Methanobactin-like RiPP Gene Cluster
Abstract
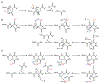
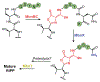
DUF692 multinuclear iron oxygenases (MNIOs) are an emerging family of tailoring enzymes involved in the biosynthesis of ribosomally synthesized and post-translationally modified peptides (RiPPs). Three members, MbnB, TglH, and ChrH, have been characterized to date and shown to catalyze unusual and complex transformations. Using a co-occurrence-based bioinformatic search strategy, we recently generated a sequence similarity network of MNIO-RiPP operons that encode one or more MNIOs adjacent to a transporter. The network revealed >1000 unique gene clusters, evidence of an unexplored biosynthetic landscape. Herein, we assess an MNIO-RiPP cluster from this network that is encoded in Proteobacteria and Actinobacteria. The cluster, which we have termed mov (for methanobactin-like operon in Vibrio), encodes a 23-residue precursor peptide, two MNIOs, a RiPP recognition element, and a transporter. Using both in vivo and in vitro methods, we show that one MNIO, homologous to MbnB, installs an oxazolone-thioamide at a Thr-Cys dyad in the precursor. Subsequently, the second MNIO catalyzes N-Cα bond cleavage of the penultimate Asn to generate a C-terminally amidated peptide. This transformation expands the reaction scope of the enzyme family, marks the first example of an MNIO-catalyzed modification that does not involve Cys, and sets the stage for future exploration of other MNIO-RiPPs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Arnison PG; Bibb MJ; Bierbaum G; Bowers AA; Bugni TS; Bulaj G; Camarero JA; Campopiano DJ; Challis GL; Clardy J; Cotter PD; Craik DJ; Dawson M; Dittmann E; Donadio S; Dorrestein PC; Entian K-D; Fischbach MA; Garavelli JS; Göransson U; Gruber CW; Haft DH; Hemscheidt TK; Hertweck C; Hill C; Horswill AR; Jaspars M; Kelly WL; Klinman JP; Kuipers OP; Link AJ; Liu W; Marahiel MA; Mitchell DA; Moll GN; Moore BS; Müller R; Nair SK; Nes IF; Norris GE; Olivera BM; Onaka H; Patchett ML; Piel J; Reaney MJT; Rebuffat S; Ross RP; Sahl H-G; Schmidt EW; Selsted ME; Severinov K; Shen B; Sivonen K; Smith L; Stein T; Süssmuth RD; Tagg JR; Tang G-L; Truman AW; Vederas JC; Walsh CT; Walton JD; Wenzel SC; Willey JM; Van Der Donk WA Ribosomally Synthesized and Post-Translationally Modified Peptide Natural Products: Overview and Recommendations for a Universal Nomenclature. Nat. Prod. Rep 2013, 30, 108–160. - PMC - PubMed
-
- Montalbán-López M; Scott TA; Ramesh S; Rahman IR; van Heel AJ; Viel JH; Bandarian V; Dittmann E; Genilloud O; Goto Y; Grande Burgos MJ; Hill C; Kim S; Koehnke J; Latham JA; Link AJ; Martínez B; Nair SK; Nicolet Y; Rebuffat S; Sahl H-G; Sareen D; Schmidt EW; Schmitt L; Severinov K; Süssmuth RD; Truman AW; Wang H; Weng J-K; van Wezel GP; Zhang Q; Zhong J; Piel J; Mitchell DA; Kuipers OP; van der Donk WA New Developments in RiPP Discovery, Enzymology and Engineering. Nat. Prod. Rep 2021, 38, 130–239. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

