Current State of Dermatology Mobile Applications With Artificial Intelligence Features
- PMID: 38452263
- PMCID: PMC10921342
- DOI: 10.1001/jamadermatol.2024.0468
Current State of Dermatology Mobile Applications With Artificial Intelligence Features
Erratum in
-
Error in Table.JAMA Dermatol. 2024 Jun 1;160(6):688. doi: 10.1001/jamadermatol.2024.1011. JAMA Dermatol. 2024. PMID: 38630467 Free PMC article. No abstract available.
-
Error in Study Type.JAMA Dermatol. 2024 Jun 1;160(6):688. doi: 10.1001/jamadermatol.2024.1342. JAMA Dermatol. 2024. PMID: 38717756 Free PMC article. No abstract available.
Abstract
Importance: With advancements in mobile technology and artificial intelligence (AI) methods, there has been a substantial surge in the availability of direct-to-consumer mobile applications (apps) claiming to aid in the assessment and management of diverse skin conditions. Despite widespread patient downloads, these apps exhibit limited evidence supporting their efficacy.
Objective: To identify and characterize current English-language AI dermatology mobile apps available for download, focusing on aspects such as purpose, supporting evidence, regulatory status, clinician input, data privacy measures, and use of image data.
Evidence review: In this cross-sectional study, both Apple and Android mobile app stores were systematically searched for dermatology-related apps that use AI algorithms. Each app's purpose, target audience, evidence-based claims, algorithm details, data availability, clinician input during development, and data usage privacy policies were evaluated.
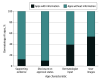
Findings: A total of 909 apps were initially identified. Following the removal of 518 duplicates, 391 apps remained. Subsequent review excluded 350 apps due to nonmedical nature, non-English languages, absence of AI features, or unavailability, ultimately leaving 41 apps for detailed analysis. The findings revealed several concerning aspects of the current landscape of AI apps in dermatology. Notably, none of the apps were approved by the US Food and Drug Administration, and only 2 of the apps included disclaimers for the lack of regulatory approval. Overall, the study found that these apps lack supporting evidence, input from clinicians and/or dermatologists, and transparency in algorithm development, data usage, and user privacy.
Conclusions and relevance: This cross-sectional study determined that although AI dermatology mobile apps hold promise for improving access to care and patient outcomes, in their current state, they may pose harm due to potential risks, lack of consistent validation, and misleading user communication. Addressing challenges in efficacy, safety, and transparency through effective regulation, validation, and standardized evaluation criteria is essential to harness the benefits of these apps while minimizing risks.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

