TARGET: A Randomized, Noninferiority Trial of a Pretest, Patient-Driven Genetic Education Webtool Versus Genetic Counseling for Prostate Cancer Germline Testing
- PMID: 38452310
- PMCID: PMC10939575
- DOI: 10.1200/PO.23.00552
TARGET: A Randomized, Noninferiority Trial of a Pretest, Patient-Driven Genetic Education Webtool Versus Genetic Counseling for Prostate Cancer Germline Testing
Abstract
Purpose: Germline genetic testing (GT) is important for prostate cancer (PCA) management, clinical trial eligibility, and hereditary cancer risk. However, GT is underutilized and there is a shortage of genetic counselors. To address these gaps, a patient-driven, pretest genetic education webtool was designed and studied compared with traditional genetic counseling (GC) to inform strategies for expanding access to genetic services.
Methods: Technology-enhanced acceleration of germline evaluation for therapy (TARGET) was a multicenter, noninferiority, randomized trial (ClinicalTrials.gov identifier: NCT04447703) comparing a nine-module patient-driven genetic education webtool versus pretest GC. Participants completed surveys measuring decisional conflict, satisfaction, and attitudes toward GT at baseline, after pretest education/counseling, and after GT result disclosure. The primary end point was noninferiority in reducing decisional conflict between webtool and GC using the validated Decisional Conflict Scale. Mixed-effects regression modeling was used to compare decisional conflict between groups. Participants opting for GT received a 51-gene panel, with results delivered to participants and their providers.
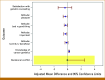
Results: The analytic data set includes primary outcome data from 315 participants (GC [n = 162] and webtool [n = 153]). Mean difference in decisional conflict score changes between groups was -0.04 (one-sided 95% CI, -∞ to 2.54; P = .01), suggesting the patient-driven webtool was noninferior to GC. Overall, 145 (89.5%) GC and 120 (78.4%) in the webtool arm underwent GT, with pathogenic variants in 15.8% (8.7% in PCA genes). Satisfaction did not differ significantly between arms; knowledge of cancer genetics was higher but attitudes toward GT were less favorable in the webtool arm.
Conclusion: The results of the TARGET study support the use of patient-driven digital webtools for expanding access to pretest genetic education for PCA GT. Further studies to optimize patient experience and evaluate them in diverse patient populations are warranted.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
This author is a member of the
No other potential conflicts of interest were reported.
Multiple regression imputation was used to replace missing data with expected values consistent with the null hypothesis of inferiority of WBGE to traditional GC. The predictors in the longitudinal mixed-effect regression models constructed to estimate the imputed decisional conflict values included study site, study group assignment, time indicator (T2
Figures

References
-
- Giri VN, Hyatt C, Gomella LG: Germline testing for men with prostate cancer: Navigating an expanding new world of genetic evaluation for precision therapy and precision management. J Clin Oncol 37:1455-1459, 2019 - PubMed
-
- de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

