Pembrolizumab Versus Placebo as Adjuvant Therapy in Resected Stage IIB or IIC Melanoma: Final Analysis of Distant Metastasis-Free Survival in the Phase III KEYNOTE-716 Study
- PMID: 38452313
- PMCID: PMC11095869
- DOI: 10.1200/JCO.23.02355
Pembrolizumab Versus Placebo as Adjuvant Therapy in Resected Stage IIB or IIC Melanoma: Final Analysis of Distant Metastasis-Free Survival in the Phase III KEYNOTE-716 Study
Abstract
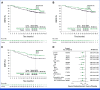
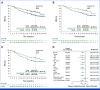
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.Pembrolizumab adjuvant therapy was shown to significantly improve recurrence-free survival (RFS) and distant metastasis-free survival (DMFS) in patients with resected stage IIB or IIC melanoma in earlier analyses of the randomized, double-blind, phase III KEYNOTE-716 study (ClinicalTrials.gov identifier: NCT03553836). We report results of the protocol-specified final analysis of DMFS for KEYNOTE-716. Overall, 976 patients were randomly allocated to pembrolizumab (n = 487) or placebo (n = 489). As of January 4, 2023, median follow-up was 39.4 months (range, 26.0-51.4 months). The median DMFS was not reached in either treatment group, and the estimated 36-month DMFS was 84.4% for pembrolizumab and 74.7% for placebo (hazard ratio [HR], 0.59 [95% CI, 0.44 to 0.79]). The median RFS was not reached in either treatment group, and the estimated 36-month RFS was 76.2% for pembrolizumab and 63.4% for placebo (HR, 0.62 [95% CI, 0.49 to 0.79]). DMFS and RFS results were consistent across most prespecified subgroups, including stage IIB and stage IIC melanoma. The safety profile of pembrolizumab was manageable and consistent with previous reports. These results continue to support the use of pembrolizumab adjuvant therapy in patients with resected stage IIB or IIC melanoma.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718–1729. - PubMed
-
- Long GV, Luke JJ, Khattak MA, et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): Distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2022;23:1378–1388. - PubMed
-
- KEYTRUDA (Pembrolizumab) Injection, for Intravenous Use [Package Insert] Rahway, NJ: Merck & Co; 2023.
-
- KEYTRUDA 25 mg/mL Concentrate for Solution for Infusion (Summary of Product Characteristics) Haarlem, the Netherlands: Merck Sharp & Dohme B.V.; 2023.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

