Oral decitabine and cedazuridine plus venetoclax for older or unfit patients with acute myeloid leukaemia: a phase 2 study
- PMID: 38452788
- PMCID: PMC12268998
- DOI: 10.1016/S2352-3026(24)00033-4
Oral decitabine and cedazuridine plus venetoclax for older or unfit patients with acute myeloid leukaemia: a phase 2 study
Abstract
Background: Hypomethylating agents combined with venetoclax are effective regimens in patients with acute myeloid leukaemia who are ineligible for intensive chemotherapy. Decitabine and cedazuridine (ASTX727) is an oral formulation of decitabine that achieves equivalent area-under-curve exposure to intravenous decitabine. We performed a single centre phase 2 study to evaluate the efficacy and safety of ASTX727 plus venetoclax.
Methods: This study enrolled patients with newly diagnosed (frontline treatment group) acute myeloid leukaemia who were ineligible for intensive chemotherapy (aged ≥75 years, an Eastern Cooperative Oncology Group [ECOG] performance status of 2-3, or major comorbidities) or relapsed or refractory acute myeloid leukaemia. Being aged 18 years or older and having an ECOG performance status of 2 or less were requirements for the relapsed or refractory disease treatment cohort, without any limits in the number of previous lines of therapy. Treatment consisted of ASTX727 (cedazuridine 100 mg and decitabine 35 mg) orally for 5 days and venetoclax 400 mg orally for 21-28 days in 28-day cycles. The primary outcome was overall response rate of ASTX727 plus venetoclax. Living patients who have not completed cycle one were not evaluable for response. Safety was analysed in all patients who started treatment. This study was registered on ClinicalTrials.gov (NCT04746235) and is ongoing. The data cutoff date for this analysis was Sept 22, 2023.
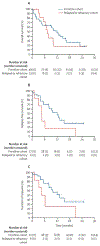
Findings: Between March 16, 2021, and Sept 18, 2023, 62 patients were enrolled (49 frontline and 13 relapsed or refractory) with a median age of 78 years (IQR 73-82). 36 (58%) were male; 53 (85%) were White, 4 (6%) Black, 2 (3%) Asian and 3 (5%) other or did not answer. 48 (77%) of 62 patients were European LeukemiaNet 2022 adverse risk, 24 (39%) had antecedent myelodysplastic syndromes, 12 (19%) had previously failed a hypomethylating agent, ten (16%) had therapy-related acute myeloid leukaemia, and 11 (18%) had TP53 mutations. The median follow-up time was 18·3 months (IQR 8·8-23·3). The overall response rate was 30 (64%) of 47 patients (95% CI 49-77) in frontline cohort and six (46%) of 13 patients (19-75) in relapsed or refractory cohort. The most common grade 3 or worse treatment-emergent adverse events were febrile neutropenia in 11 (18%) of 62 patients, pneumonia in eight (13%), respiratory failure in five (8%), bacteraemia in four (6%), and sepsis in four (6%). Three deaths occurred in patients in remission (one sepsis, one gastrointestinal haemorrhage, and one respiratory failure) and were potentially treatment related.
Interpretation: ASTX727 plus venetoclax is an active fully oral regimen and safe in most older or unfit patients with acute myeloid leukaemia. Our findings should be confirmed in larger multicentric studies.
Funding: MD Anderson Cancer Center Support Grant, Myelodysplastic Syndrome/Acute Myeloid Leukaemia Moon Shot, Leukemia SPORE, Taiho Oncology, and Astex Pharmaceuticals.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests GG-M declares grants from Astex, Novartis, AbbVie, Genentech, Aprea, Curis, and Gilead; consulting fees from Astex, Acceleron, and BMS; and payment or honoraria from Astex, Acceleron, AbbVie, Gilead, Curis, Genentech, and BMS. NS declares research funding from Takeda Oncology, Astellas Pharma, Xencor, Stemline Therapeutics, and NextCure; consulting fees from Pfizer, GlaxoSmithKline, NKARTA, Autolus, and Sanofi; and payment or honoraria from Adaptive Biotechnologies, Novartis, Amgen, Takeda Oncology, Pfizer, Astellas Pharma, Sanofi, and BeiGene. GI declares grants from Celgene, Kura Oncology, Syndax, Merck, Cullinan, and Novartis and consulting fees from Novartis, Kura Oncology, and Nuprobe. NJ declares research funding from Pharmacyclics, AbbVie, Genentech, AstraZeneca, BMS, Pfizer, ADC Therapeutics, Cellectis, Adaptive Biotechnologies, Precision Biosciences, Fate Therapeutics, Kite and Gilead, Mingsight, Takeda, Medisix, Loxo Oncology, Novalgen, Dialectic Therapeutics, Newave, TransThera Sciences, Novartis, Carna Biosciences, Sana Biotechnology, and Kisoji Biotechnology and honoraria or advisory board participation from Pharmacyclics, Janssen, AbbVie, Genentech, AstraZeneca, BMS, Adaptive Biotechnologies, Kite and Gilead, Precision Biosciences, Beigene, Cellectis, MEI Pharma, Ipsen, CareDX, MingSight, and Novalgen. AM declares research funding from Lin Biosciences and Chimeric Therapeutics and travel support from Cero Bio. EJ declares grants from AbbVie, Adaptive Biotechnologies, Amgen, Pfizer, and Takeda and consulting fees from AbbVie, Adaptive Biotechnologies, Amgen, BMS, Genentech, Incyte, Novartis, Pfizer, and Takeda. CDD declares grants from AbbVie, Astex, ImmuneOnc, BMS, Cleave, Foghorn, Loxo, Rigel, and Servier; consulting fees from Amgen, AbbVie, Astellas, BMS, Genmab, GSK, Gilead, Jazz, Shrodinger, Servier, and Stemline; payment or honoraria from AbbVie, Astellas, BMS, Jazz, and Servier; travel support from Servier; and participation on a data safety board for Genmab. TK declares grants from BMS, AbbVie, Amgen, Ascentage Pharma Group, Astellas Pharma, DrenBio, Astex, AstraZeneca, BMS, Celgene, Incyte, Cellenkos, Cyclacel, Delta-Fly Pharma, Genentech, Genfleet, Glycomimetics, Iterion, Janssen, Jazz Pharmaceuticals, Pfizer, Pulmotect, Regeneron, and SELLAS; consulting fees from AbbVie, Agios, Daiichi Sankyo, Genentech, Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Pulmotect, Sanofi-Aventis, and Servier; and payment or honoraria from AbbVie, Agios, Daiichi Sankyo, DAVA Oncology, Delta-Fly, DrenBio, Genentech, Genfleet, Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, Rigel, Sanofi-Aventis, SELLAS, and Servier. ND declares grants from Daiichi-Sankyo, BMS, Pfizer, Gilead, Servier, Genentech, Astellas, AbbVie, ImmunoGen, Amgen, Trillium, Hanmi, Trovagene, FATE Therapeutics, Novimmune, Glycomimetics, and KITE and consulting fees from Daiichi-Sankyo, BMS, Pfizer, Gilead, Servier, Genentech, Astellas, AbbVie, ImmunoGen, Amgen, Trillium, Arog, Novartis, Jazz, Celgene, Syndax, Shattuck Labs, Agios, KITE, and Stemline and Menarini. MK declares grants from AbbVie, Genentech, Gilead, ImmunoGen, MEI Pharma, Precision Biosciences, Rafael Pharmaceutical, Sanofi-Aventis, and Stemline Therapeutics; consulting fees from AbbVie, AstraZeneca, Boehringer, Hoffman-La Roche, Genentech, Gilead, Janssen, Legend Biotech, MEI Pharma, Redona, Sanofi-Aventis, Sellas, Stemline Therapeutics, and Vincerx; participation on an advisory board for AbbVie, Auxenion, GmbH, Bakx Therapeutics, Dark Blue Therapeutics, Hoffman-La Roche, Genentech, Gilead, Stemline Therapeutics, and Vincerx; has a board of directors role for Immune Oncology; clinical trial support from AbbVie, AstraZeneca, Cellectis, Genentech, Janssen, Pfizer, Sanofi-Aventis, and Stemline Therapeutics; and intellectual property from Reata Pharmaceuticals. HK declares grants from AbbVie, Amgen, Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz, and Novartis and payment or honoraria from AbbVie, Amgen, Amphista, Ascentage, Astellas, Biologix, Curis, Ipsen Biopharmaceuticals, KAHR Medical, Labcorp, Novartis, Pfizer, Shenzhen Target Rx, Stemline, and Takeda. FR declares clinical trial support from Astex and Taiho Oncology and payment or honoraria from Taiho Oncology. All other authors declare no competing interests.
Figures


Similar articles
-
Oral decitabine and cedazuridine maintenance after haematopoietic stem-cell transplantation in very high-risk acute myeloid leukaemia or myelodysplastic syndrome (GFM-DACORAL-DLI): a multicentre, single-arm, phase 2 trial.Lancet Haematol. 2025 Aug 7:S2352-3026(25)00172-3. doi: 10.1016/S2352-3026(25)00172-3. Online ahead of print. Lancet Haematol. 2025. PMID: 40784355
-
Reduced dose azacitidine plus venetoclax as maintenance therapy in acute myeloid leukaemia following intensive or low-intensity induction: a single-centre, single-arm, phase 2 trial.Lancet Haematol. 2024 Apr;11(4):e287-e298. doi: 10.1016/S2352-3026(24)00034-6. Lancet Haematol. 2024. PMID: 38548404 Free PMC article. Clinical Trial.
-
Bexmarilimab plus azacitidine for high-risk myelodysplastic syndrome and relapsed or refractory acute myeloid leukaemia: results from the dose-escalation part of a multicentre, single-arm, phase 1/2 trial.Lancet Haematol. 2025 Jul;12(7):e516-e528. doi: 10.1016/S2352-3026(25)00103-6. Epub 2025 May 28. Lancet Haematol. 2025. PMID: 40449509 Clinical Trial.
-
Thrombopoietin mimetics for patients with myelodysplastic syndromes.Cochrane Database Syst Rev. 2017 Sep 30;9(9):CD009883. doi: 10.1002/14651858.CD009883.pub2. Cochrane Database Syst Rev. 2017. PMID: 28962071 Free PMC article.
-
The efficacy and safety of venetoclax combined with decitabine in elderly patients with acute myeloid leukemia: a systematic review and meta-analysis.Clin Exp Med. 2025 Jul 9;25(1):239. doi: 10.1007/s10238-025-01794-w. Clin Exp Med. 2025. PMID: 40632299 Free PMC article.
Cited by
-
Updates on Therapy Options in Fit and Unfit Patients with Newly Diagnosed AML.Curr Treat Options Oncol. 2025 Aug 23. doi: 10.1007/s11864-025-01351-3. Online ahead of print. Curr Treat Options Oncol. 2025. PMID: 40848224 Review.
-
Contemporary outcomes of octa-nonagenarians with newly diagnosed acute myeloid leukemia.Cancer. 2025 Aug 15;131(16):e70028. doi: 10.1002/cncr.70028. Cancer. 2025. PMID: 40772501 Free PMC article.
-
Comprehensive view on chemotherapy-free management of acute myeloid leukemia by using venetoclax in combination with targeted and/or immune therapies.Cell Death Discov. 2025 Aug 13;11(1):379. doi: 10.1038/s41420-025-02678-4. Cell Death Discov. 2025. PMID: 40804253 Free PMC article. Review.
-
The association of FAT1 mutations with therapeutic outcomes in AML, especially in receiving venetoclax combination.Front Oncol. 2025 Mar 31;15:1456832. doi: 10.3389/fonc.2025.1456832. eCollection 2025. Front Oncol. 2025. PMID: 40242237 Free PMC article.
-
Epigenetics-targeted drugs: current paradigms and future challenges.Signal Transduct Target Ther. 2024 Nov 26;9(1):332. doi: 10.1038/s41392-024-02039-0. Signal Transduct Target Ther. 2024. PMID: 39592582 Free PMC article. Review.
References
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med 2015; 373: 1136–52. - PubMed
-
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2018. 2021. https://seer.cancer.gov/csr/1975_2018/ (accessed Sept 30, 2023).
-
- Hulegårdh E, Nilsson C, Lazarevic V, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol 2015; 90: 208–14. - PubMed
-
- Tsai CH, Hou HA, Tang JL, et al. Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia 2016; 30: 1485–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

