Iron-sulfur cluster assembly scaffold protein IscU is required for activation of ferric uptake regulator (Fur) in Escherichiacoli
- PMID: 38452854
- PMCID: PMC11001641
- DOI: 10.1016/j.jbc.2024.107142
Iron-sulfur cluster assembly scaffold protein IscU is required for activation of ferric uptake regulator (Fur) in Escherichiacoli
Abstract
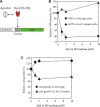
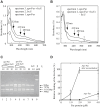
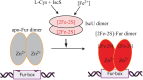
It was generally postulated that when intracellular free iron content is elevated in bacteria, the ferric uptake regulator (Fur) binds its corepressor a mononuclear ferrous iron to regulate intracellular iron homeostasis. However, the proposed iron-bound Fur had not been identified in any bacteria. In previous studies, we have demonstrated that Escherichia coli Fur binds a [2Fe-2S] cluster in response to elevation of intracellular free iron content and that binding of the [2Fe-2S] cluster turns on Fur as an active repressor to bind a specific DNA sequence known as the Fur-box. Here we find that the iron-sulfur cluster assembly scaffold protein IscU is required for the [2Fe-2S] cluster assembly in Fur, as deletion of IscU inhibits the [2Fe-2S] cluster assembly in Fur and prevents activation of Fur as a repressor in E. coli cells in response to elevation of intracellular free iron content. Additional studies reveal that IscU promotes the [2Fe-2S] cluster assembly in apo-form Fur and restores its Fur-box binding activity in vitro. While IscU is also required for the [2Fe-2S] cluster assembly in the Haemophilus influenzae Fur in E. coli cells, deletion of IscU does not significantly affect the [2Fe-2S] cluster assembly in the E. coli ferredoxin and siderophore-reductase FhuF. Our results suggest that IscU may have a unique role for the [2Fe-2S] cluster assembly in Fur and that regulation of intracellular iron homeostasis is closely coupled with iron-sulfur cluster biogenesis in E. coli.
Keywords: ferric uptake regulator (Fur); iron homeostasis; iron–sulfur cluster biogenesis.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Ferric uptake regulator (Fur) reversibly binds a [2Fe-2S] cluster to sense intracellular iron homeostasis in Escherichia coli.J Biol Chem. 2020 Nov 13;295(46):15454-15463. doi: 10.1074/jbc.RA120.014814. Epub 2020 Sep 14. J Biol Chem. 2020. PMID: 32928958 Free PMC article.
-
Ferric uptake regulator (Fur) binds a [2Fe-2S] cluster to regulate intracellular iron homeostasis in Escherichia coli.J Biol Chem. 2023 Jun;299(6):104748. doi: 10.1016/j.jbc.2023.104748. Epub 2023 Apr 24. J Biol Chem. 2023. PMID: 37100285 Free PMC article.
-
The C-terminal domain of the ferric uptake regulator (Fur) binds a [2Fe-2S] cluster to sense the intracellular free iron content in Escherichia coli.Biometals. 2023 Dec;36(6):1285-1294. doi: 10.1007/s10534-023-00517-6. Epub 2023 Jun 21. Biometals. 2023. PMID: 37344741
-
Members of the Fur protein family regulate iron and zinc transport in E. coli and characteristics of the Fur-regulated fhuF protein.J Mol Microbiol Biotechnol. 2002 May;4(3):217-22. J Mol Microbiol Biotechnol. 2002. PMID: 11931550 Review.
-
Metamorphic protein IscU alternates conformations in the course of its role as the scaffold protein for iron-sulfur cluster biosynthesis and delivery.FEBS Lett. 2013 Apr 17;587(8):1172-9. doi: 10.1016/j.febslet.2013.01.003. Epub 2013 Jan 16. FEBS Lett. 2013. PMID: 23333622 Free PMC article. Review.
References
-
- Sevilla E., Bes M.T., Peleato M.L., Fillat M.F. Fur-like proteins: beyond the ferric uptake regulator (Fur) paralog. Arch. Biochem. Biophys. 2021;701 - PubMed
-
- McHugh J.P., Rodríguez-Quiñones F., Abdul-Tehrani H., Svistunenko D.A., Poole R.K., Cooper C.E., et al. Global iron-dependent gene regulation in Escherichia coli: a new mechanism for iron homeostasis. J. Biol. Chem. 2003;278:29478–29486. - PubMed
-
- Abed N., Bickle M., Mari B., Schapira M., Sanjuan-Espana R., Robbe Sermesant K., et al. A comparative analysis of perturbations caused by a gene knock-out, a dominant negative allele, and a set of peptide aptamers. Mol. Cell Proteomics. 2007;6:2110–2121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

