Urinary Plasminogen as a Marker of Disease Progression in Human Glomerular Disease
- PMID: 38452919
- PMCID: PMC11260534
- DOI: 10.1053/j.ajkd.2024.01.520
Urinary Plasminogen as a Marker of Disease Progression in Human Glomerular Disease
Abstract
Rationale & objective: Glomerular disorders have a highly variable clinical course, and biomarkers that reflect the molecular mechanisms underlying their progression are needed. Based on our previous work identifying plasminogen as a direct cause of podocyte injury, we designed this study to test the association between urine plasmin(ogen) (ie, plasmin and its precursor plasminogen) and end-stage kidney disease (ESKD).
Study design: Multicenter cohort study.
Setting & participants: 1,010 patients enrolled in the CureGN Cohort with biopsy-proven glomerular disease (focal segmental glomerulosclerosis, membranous nephropathy, and immunoglobulin A nephropathy).
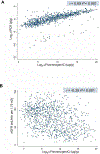
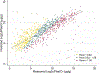
Predictors: The main predictor was urine plasmin(ogen) at baseline. Levels were measured by an electrochemiluminescent immunoassay developed de novo. Traditional clinical and analytical characteristics were used for adjustment. The ratio of urine plasmin(ogen)/expected plasmin(ogen) was evaluated as a predictor in a separate model.
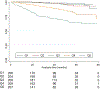
Outcome: Progression to ESKD.
Analytical approach: Cox regression was used to examine the association between urinary plasmin(ogen) and time to ESKD. Urinary markers were log2 transformed to approximate normal distribution and normalized to urinary creatinine (Log2uPlasminogen/cr, Log2 urinary protein/cr [UPCR]). Expected plasmin(ogen) was calculated by multiple linear regression.
Results: Adjusted Log2uPlasminogen/cr was significantly associated with ESKD (HR per doubling Log2 uPlasminogen/cr 1.31 [95% CI, 1.22-1.40], P<0.001). Comparison of the predictive performance of the models including Log2 uPlasminogen/cr, Log2 UPCR, or both markers showed the plasmin(ogen) model superiority. The ratio of measured/expected urine plasmin(ogen) was independently associated with ESKD: HR, 0.41 (95% CI, 0.22-0.77) if ratio<0.8 and HR 2.42 (95% CI, 1.54-3.78) if ratio>1.1 (compared with ratio between 0.8 and 1.1).
Limitations: Single plasmin(ogen) determination does not allow for the study of changes over time. The use of a cohort of mostly white patients and the restriction to patients with 3 glomerular disorders limits the external validity of our analysis.
Conclusions: Urinary plasmin(ogen) and the ratio of measured/expected plasmin(ogen) are independently associated with ESKD in a cohort of patients with glomerular disease. Taken together with our previous experimental findings, urinary plasmin(ogen) could be a useful biomarker in prognostic decision making and a target for the development of novel therapies in patients with proteinuria and glomerular disease.
Plain-language summary: Glomerular diseases are an important cause of morbidity and mortality in patients of all ages. Knowing the individual risk of progression to dialysis or transplantation would help to plan the follow-up and treatment of these patients. Our work studies the usefulness of urinary plasminogen as a marker of progression in this context, since previous studies indicate that plasminogen may be involved in the mechanisms responsible for the progression of these disorders. Our work in a sample of 1,010 patients with glomerular disease demonstrates that urinary plasminogen (as well as the ratio of measured to expected plasminogen) is associated with the risk of progression to end-stage kidney disease. Urine plasminogen exhibited good performance and, if further validated, could enable risk stratification for timely interventions in patients with proteinuria and glomerular disease.
Keywords: Biomarkers; end-stage kidney disease; glomerular disease; urinary plasminogen.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Kidney Disease: Improving Global Outcomes Glomerular Diseases Work G. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4S): S1–S276. - PubMed
-
- Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71(12): 1205–1214. - PubMed
-
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389(10075): 1238–1252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

