The effects of metabolism on the immune microenvironment in colorectal cancer
- PMID: 38453888
- PMCID: PMC10920911
- DOI: 10.1038/s41420-024-01865-z
The effects of metabolism on the immune microenvironment in colorectal cancer
Abstract
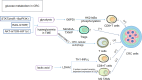
Colorectal cancer (CRC) is a malignancy that is widely prevalent worldwide. Due to its unsatisfactory treatment outcome and extremely poor prognosis, many studies on the molecular mechanisms and pathological mechanisms of CRC have been published in recent years. The tumor microenvironment (TME) is an extremely important feature of tumorigenesis and one of the hallmarks of tumor development. Metabolic reprogramming is currently a hot topic in tumor research, and studies on this topic have provided important insights into CRC development. In particular, metabolic reprogramming in cancer causes changes in the composition of energy and nutrients in the TME. Furthermore, it can alter the complex crosstalk between immune cells and associated immune factors, such as associated macrophages and T cells, which play important immune roles in the TME, in turn affecting the immune escape of tumors by altering immune surveillance. In this review, we summarize several metabolism-related processes affecting the immune microenvironment of CRC tumors. Our results showed that the immune microenvironment is regulated by metabolic reprogramming and influences the development of CRC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68:394–424. - PubMed
Publication types
Grants and funding
- 82070536/National Natural Science Foundation of China (National Science Foundation of China)
- 82073087/National Natural Science Foundation of China (National Science Foundation of China)
- 82160505/National Natural Science Foundation of China (National Science Foundation of China)
- 81660098/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

