TREM2 deficiency impairs the energy metabolism of Schwann cells and exacerbates peripheral neurological deficits
- PMID: 38453910
- PMCID: PMC10920707
- DOI: 10.1038/s41419-024-06579-9
TREM2 deficiency impairs the energy metabolism of Schwann cells and exacerbates peripheral neurological deficits
Abstract
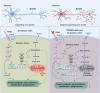
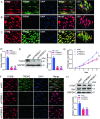
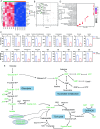
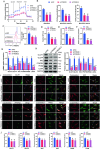
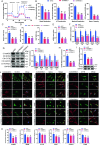
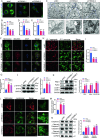
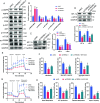
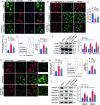
Triggering receptor expressed on myeloid cells-2 (TREM2) has been implicated in susceptibility to neurodegenerative disease. Schwann cells (SCs), the predominant glial cell type in the peripheral nervous system (PNS), play a crucial role in myelination, providing trophic support for neurons and nerve regeneration. However, the function of TREM2 in SCs has not been fully elucidated. Here, we found that TREM2 is expressed in SCs but not in neurons in the PNS. TREM2 deficiency leads to disruption of glycolytic flux and oxidative metabolism in SCs, impairing cell proliferation. The energy crisis caused by TREM2 deficiency triggers mitochondrial damage and autophagy by activating AMPK and impairing PI3K-AKT-mTOR signaling. Combined metabolomic analysis demonstrated that energic substrates and energy metabolic pathways were significantly impaired in TREM2-deficient SCs. Moreover, TREM2 deficiency impairs energy metabolism and axonal growth in sciatic nerve, accompanied by exacerbation of neurological deficits and suppression of nerve regeneration in a mouse model of acute motor axonal neuropathy. These results indicate that TREM2 is a critical regulator of energy metabolism in SCs and exerts neuroprotective effects on peripheral neuropathy. TREM2 deficiency impairs glycolysis and oxidative metabolism in Schwann cells, resulting in compromised cell proliferation. The energy crisis caused by TREM2 deficiency induces mitochondrial damage and autophagy by activating AMPK and impairing PI3K-AKT-mTOR signaling. Moreover, TREM2 deficiency disrupts the energy metabolism of the sciatic nerve and impairs support for axonal regeneration, accompanied by exacerbation of neurological deficits and suppression of nerve regeneration in a mouse model of acute motor axonal neuropathy (by FigDraw).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

