Malaria blood stage infection suppresses liver stage infection via host-induced interferons but not hepcidin
- PMID: 38453916
- PMCID: PMC10920859
- DOI: 10.1038/s41467-024-46270-3
Malaria blood stage infection suppresses liver stage infection via host-induced interferons but not hepcidin
Abstract
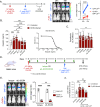
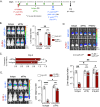
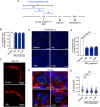
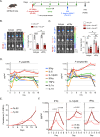
Malaria-causing Plasmodium parasites first replicate as liver stages (LS), which then seed symptomatic blood stage (BS) infection. Emerging evidence suggests that these stages impact each other via perturbation of host responses, and this influences the outcome of natural infection. We sought to understand whether the parasite stage interplay would affect live-attenuated whole parasite vaccination, since the efficacy of whole parasite vaccines strongly correlates with their extend of development in the liver. We thus investigated the impact of BS infection on LS development of genetically attenuated and wildtype parasites in female rodent malaria models and observed that for both, LS infection suffered severe suppression during concurrent BS infection. Strikingly and in contrast to previously published studies, we find that the BS-induced iron-regulating hormone hepcidin is not mediating suppression of LS development. Instead, we demonstrate that BS-induced host interferons are the main mediators of LS developmental suppression. The type of interferon involved depended on the BS-causing parasite species. Our study provides important mechanistic insights into the BS-mediated suppression of LS development. This has direct implications for understanding the outcomes of live-attenuated Plasmodium parasite vaccination in malaria-endemic areas and might impact the epidemiology of natural malaria infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
RNA-Seq Analysis Illuminates the Early Stages of Plasmodium Liver Infection.mBio. 2020 Feb 4;11(1):e03234-19. doi: 10.1128/mBio.03234-19. mBio. 2020. PMID: 32019802 Free PMC article.
-
Vaccination with chemically attenuated Plasmodium falciparum asexual blood-stage parasites induces parasite-specific cellular immune responses in malaria-naïve volunteers: a pilot study.BMC Med. 2018 Oct 8;16(1):184. doi: 10.1186/s12916-018-1173-9. BMC Med. 2018. PMID: 30293531 Free PMC article. Clinical Trial.
-
In silico identification of genetically attenuated vaccine candidate genes for Plasmodium liver stage.Infect Genet Evol. 2015 Dec;36:72-81. doi: 10.1016/j.meegid.2015.09.002. Epub 2015 Sep 6. Infect Genet Evol. 2015. PMID: 26348884
-
Arrested Plasmodium liver stages as experimental anti-malaria vaccines.Hum Vaccin. 2011 Jan-Feb;7 Suppl:16-21. doi: 10.4161/hv.7.0.14557. Epub 2011 Jan 1. Hum Vaccin. 2011. PMID: 21266857 Review.
-
Genetic engineering of attenuated malaria parasites for vaccination.Curr Opin Biotechnol. 2012 Dec;23(6):908-16. doi: 10.1016/j.copbio.2012.04.003. Epub 2012 May 4. Curr Opin Biotechnol. 2012. PMID: 22560204 Review.
Cited by
-
Elimination of intra-hepatocytic malaria parasites is driven by non-canonical autophagy but not nitric oxide production.iScience. 2025 Mar 1;28(4):112052. doi: 10.1016/j.isci.2025.112052. eCollection 2025 Apr 18. iScience. 2025. PMID: 40463965 Free PMC article.
-
mRNA-LNP vaccine encoding the Plasmodium vivax circumsporozoite protein is highly immunogenic and confers protection in mice.Mol Ther Nucleic Acids. 2025 Jul 30;36(3):102645. doi: 10.1016/j.omtn.2025.102645. eCollection 2025 Sep 9. Mol Ther Nucleic Acids. 2025. PMID: 40832631 Free PMC article.
-
Cutaneous leishmaniasis and iron metabolism: current insights and challenges.Front Immunol. 2024 Dec 3;15:1488590. doi: 10.3389/fimmu.2024.1488590. eCollection 2024. Front Immunol. 2024. PMID: 39691716 Free PMC article. Review.
References
-
- Organization W. H. World Malaria Report 2021 (World Health Organisation, 2021).
-
- Chora, Â. F., Mota, M. M. & Prudêncio, M. The reciprocal influence of the liver and blood stages of the malaria parasite’s life cycle. Int. J. Parasitol. 52, 711–715 (2022). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

