Canonical and non-canonical integrin-based adhesions dynamically interconvert
- PMID: 38453931
- PMCID: PMC10920918
- DOI: 10.1038/s41467-024-46381-x
Canonical and non-canonical integrin-based adhesions dynamically interconvert
Abstract
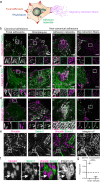
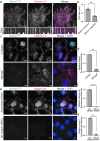
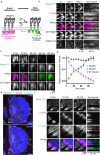
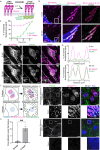
Adhesions are critical for anchoring cells in their environment, as signaling platforms and for cell migration. In line with these diverse functions different types of cell-matrix adhesions have been described. Best-studied are the canonical integrin-based focal adhesions. In addition, non-canonical integrin adhesions lacking focal adhesion proteins have been discovered. These include reticular adhesions also known as clathrin plaques or flat clathrin lattices, that are enriched in clathrin and other endocytic proteins, as well as extensive adhesion networks and retraction fibers. How these different adhesion types that share a common integrin backbone are related and whether they can interconvert is unknown. Here, we identify the protein stonin1 as a marker for non-canonical αVβ5 integrin-based adhesions and demonstrate by live cell imaging that canonical and non-canonical adhesions can reciprocally interconvert by the selective exchange of components on a stable αVβ5 integrin scaffold. Hence, non-canonical adhesions can serve as points of origin for the generation of canonical focal adhesions.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Focal adhesions, reticular adhesions, flat clathrin lattices: what divides them, what unites them?Am J Physiol Cell Physiol. 2025 Jan 1;328(1):C288-C302. doi: 10.1152/ajpcell.00821.2024. Epub 2024 Dec 9. Am J Physiol Cell Physiol. 2025. PMID: 39652817 Review.
-
Reticular adhesions are assembled at flat clathrin lattices and opposed by active integrin α5β1.J Cell Biol. 2023 Aug 7;222(8):e202303107. doi: 10.1083/jcb.202303107. Epub 2023 May 26. J Cell Biol. 2023. PMID: 37233325 Free PMC article.
-
Curved adhesions mediate cell attachment to soft matrix fibres in three dimensions.Nat Cell Biol. 2023 Oct;25(10):1453-1464. doi: 10.1038/s41556-023-01238-1. Epub 2023 Sep 28. Nat Cell Biol. 2023. PMID: 37770566 Free PMC article.
-
Podosome-type adhesions and focal adhesions, so alike yet so different.Eur J Cell Biol. 2008 Sep;87(8-9):491-506. doi: 10.1016/j.ejcb.2008.02.012. Epub 2008 Apr 15. Eur J Cell Biol. 2008. PMID: 18417250 Review.
-
Reticular adhesions are a distinct class of cell-matrix adhesions that mediate attachment during mitosis.Nat Cell Biol. 2018 Nov;20(11):1290-1302. doi: 10.1038/s41556-018-0220-2. Epub 2018 Oct 22. Nat Cell Biol. 2018. PMID: 30361699
Cited by
-
Mechanisms and Therapeutic Strategies for Minority Cell-Induced Paclitaxel Resistance and Tumor Progression Mediated by Mechanical Forces.Adv Sci (Weinh). 2025 Jun;12(22):e2417805. doi: 10.1002/advs.202417805. Epub 2025 Apr 24. Adv Sci (Weinh). 2025. PMID: 40270447 Free PMC article.
-
Crosstalk of growth factor receptors at plasma membrane clathrin-coated sites.bioRxiv [Preprint]. 2024 May 18:2024.05.16.594559. doi: 10.1101/2024.05.16.594559. bioRxiv. 2024. PMID: 38903101 Free PMC article. Preprint.
-
Spatial and signaling overlap of growth factor receptor systems at clathrin-coated sites.Mol Biol Cell. 2024 Nov 1;35(11):ar138. doi: 10.1091/mbc.E24-05-0226. Epub 2024 Sep 18. Mol Biol Cell. 2024. PMID: 39292879 Free PMC article.

