Alpha5 nicotine acetylcholine receptor subunit promotes intrahepatic cholangiocarcinoma metastasis
- PMID: 38453934
- PMCID: PMC10920868
- DOI: 10.1038/s41392-024-01761-z
Alpha5 nicotine acetylcholine receptor subunit promotes intrahepatic cholangiocarcinoma metastasis
Abstract
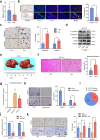
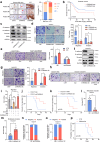
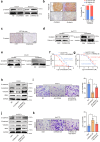
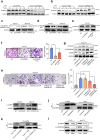
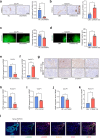
Neurotransmitter-initiated signaling pathway were reported to play an important role in regulating the malignant phenotype of tumor cells. Cancer cells could exhibit a "neural addiction" property and build up local nerve networks to achieve an enhanced neurotransmitter-initiated signaling through nerve growth factor-mediated axonogenesis. Targeting the dysregulated nervous systems might represent a novel strategy for cancer treatment. However, whether intrahepatic cholangiocarcinoma (ICC) could build its own nerve networks and the role of neurotransmitters in the progression ICC remains largely unknown. Immunofluorescence staining and Enzyme-linked immunosorbent assay suggested that ICC cells and the infiltrated nerves could generate a tumor microenvironment rich in acetylcholine that promotes ICC metastasis by inducing epithelial-mesenchymal transition (EMT). Acetylcholine promoted ICC metastasis through interacting with its receptor, alpha 5 nicotine acetylcholine receptor subunits (CHRNA5). Furthermore, acetylcholine/CHRNA5 axis activated GSK3β/β-catenin signaling pathway partially through the influx of Ca2+-mediated activation of Ca/calmodulin-dependent protein kinases (CAMKII). In addition, acetylcholine signaling activation also expanded nerve infiltration through increasing the expression of Brain-Derived Neurotrophic Factor (BDNF), which formed a feedforward acetylcholine-BDNF axis to promote ICC progression. KN93, a small-molecule inhibitor of CAMKII, significantly inhibited the migration and enhanced the sensitivity to gemcitabine of ICC cells. Above all, Acetylcholine/CHRNA5 axis increased the expression of β-catenin to promote the metastasis and resistance to gemcitabine of ICC via CAMKII/GSK3β signaling, and the CAMKII inhibitor KN93 may be an effective therapeutic strategy for combating ICC metastasis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Liebig C, et al. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 91959203/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 91959203/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 91959203/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 91959203/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 91959203/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 91959203/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 91959203/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
- 91959203/National Natural Science Foundation of China (National Science Foundation of China)
- 81930074/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

