Efficient retrosynthetic planning with MCTS exploration enhanced A* search
- PMID: 38454002
- PMCID: PMC10920677
- DOI: 10.1038/s42004-024-01133-2
Efficient retrosynthetic planning with MCTS exploration enhanced A* search
Abstract
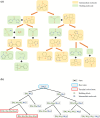
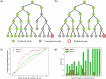
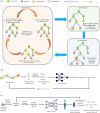
Retrosynthetic planning, which aims to identify synthetic pathways for target molecules from starting materials, is a fundamental problem in synthetic chemistry. Computer-aided retrosynthesis has made significant progress, in which heuristic search algorithms, including Monte Carlo Tree Search (MCTS) and A* search, have played a crucial role. However, unreliable guiding heuristics often cause search failure due to insufficient exploration. Conversely, excessive exploration also prevents the search from reaching the optimal solution. In this paper, MCTS exploration enhanced A* (MEEA*) search is proposed to incorporate the exploratory behavior of MCTS into A* by providing a look-ahead search. Path consistency is adopted as a regularization to improve the generalization performance of heuristics. Extensive experimental results on 10 molecule datasets demonstrate the effectiveness of MEEA*. Especially, on the widely used United States Patent and Trademark Office (USPTO) benchmark, MEEA* achieves a 100.0% success rate. Moreover, for natural products, MEEA* successfully identifies bio-retrosynthetic pathways for 97.68% test compounds.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Retrosynthetic planning with experience-guided Monte Carlo tree search.Commun Chem. 2023 Jun 10;6(1):120. doi: 10.1038/s42004-023-00911-8. Commun Chem. 2023. PMID: 37301940 Free PMC article.
-
Comparing search algorithms on the retrosynthesis problem.Mol Inform. 2024 Jul;43(7):e202300259. doi: 10.1002/minf.202300259. Epub 2024 Jun 12. Mol Inform. 2024. PMID: 38864849
-
A self-learning Monte Carlo tree search algorithm for robot path planning.Front Neurorobot. 2023 Jul 6;17:1039644. doi: 10.3389/fnbot.2023.1039644. eCollection 2023. Front Neurorobot. 2023. PMID: 37483541 Free PMC article.
-
Path planning optimization in unmanned aerial vehicles using meta-heuristic algorithms: a systematic review.Environ Monit Assess. 2022 Oct 25;195(1):30. doi: 10.1007/s10661-022-10590-y. Environ Monit Assess. 2022. PMID: 36282405
-
Natural Product Synthesis through the Lens of Informatics.Acc Chem Res. 2021 Mar 2;54(5):1157-1167. doi: 10.1021/acs.accounts.0c00791. Epub 2021 Feb 12. Acc Chem Res. 2021. PMID: 33577292 Free PMC article. Review.
Cited by
-
Discovery, design, and engineering of enzymes based on molecular retrobiosynthesis.mLife. 2025 Mar 28;4(2):107-125. doi: 10.1002/mlf2.70009. eCollection 2025 Apr. mLife. 2025. PMID: 40313979 Free PMC article. Review.
-
Cross-disciplinary perspectives on the potential for artificial intelligence across chemistry.Chem Soc Rev. 2025 Jun 3;54(11):5433-5469. doi: 10.1039/d5cs00146c. Chem Soc Rev. 2025. PMID: 40278836 Free PMC article. Review.
-
Enhancing Monte Carlo Tree Search for Retrosynthesis.J Chem Inf Model. 2025 Jul 14;65(13):6537-6546. doi: 10.1021/acs.jcim.5c00417. Epub 2025 Jun 13. J Chem Inf Model. 2025. PMID: 40512567 Free PMC article.
References
-
- Corey EJ. The logic of chemical synthesis: multistep synthesis of complex carbogenic molecules. Angew. Chem. Int. Ed. Engl. 1991;30:455–465. doi: 10.1002/anie.199104553. - DOI
-
- Yan C, et al. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018;3:1–19. doi: 10.1038/natrevmats.2018.3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

