Distribution of exchangeable Ca2+ during the process of Larix decidua Mill. pollination and germination
- PMID: 38454044
- PMCID: PMC10920793
- DOI: 10.1038/s41598-024-54903-2
Distribution of exchangeable Ca2+ during the process of Larix decidua Mill. pollination and germination
Abstract
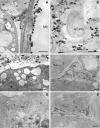
The involvement of Ca2+ ions in angiosperms sexual processes is well established, while in gymnosperms, such knowledge remains limited and is still a topic of discussion. In this study, we focused on Larix decidua, using Alizarin-red S staining and the pyroantimonate method to examine the tissue and subcellular distribution of free and loosely bound Ca2+ ions at different stages of the male gametophyte's development and its interaction with the ovule. Our findings show that in larch, both the germination of pollen grains and the growth of pollen tubes occur in an environment rich in Ca2+. These ions play a crucial role in the adhesion of the pollen grain to the stigmatic tip and its subsequent movement to the micropylar canal. There is a significant presence of free and loosely bound Ca2+ ions in both the fluid of the micropylar canal and the extracellular matrix of the nucellus. As the pollen tube extends through the nucellus, we observed a notable accumulation of Ca2+ ions just above the entry to the mature archegonium, a region likely crucial for the male gametophyte's directional growth. Meanwhile, the localized presence of free and loosely bound Ca2+ ions within the egg cell cytoplasm may inhibit the pollen tubes growth and rupture, playing an important role in fertilization.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Villar M, Knox R, Dumas C. Effective pollination period and nature of pollen-collecting apparatus in the gymnosperm, Larix leptolepis. Ann. Bot. 1984;53:279–284. doi: 10.1093/oxfordjournals.aob.a086689. - DOI
-
- Rodkiewicz, B. The Embryology of Gymnosperms (Państwowe Wydawnictwo Naukowe, 1984) [in Polish].
-
- Owens JN, Morris SJ, Catalano GL. How the pollination mechanism and prezygotic and postzygotic events affect seed production in Larix occidentalis. Can. J. For. Res. 1994;24:917–927. doi: 10.1139/x94-121. - DOI
-
- Takaso T, Owens JN. Pollen movement in the micropylar canal of Larix and its simulation. J. Plant Res. 1997;110:259–264. doi: 10.1007/BF02509314. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

