Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial
- PMID: 38454126
- PMCID: PMC11031404
- DOI: 10.1038/s41591-024-02875-1
Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial
Erratum in
-
Author Correction: Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial.Nat Med. 2024 May;30(5):1501. doi: 10.1038/s41591-024-02928-5. Nat Med. 2024. PMID: 38514871 Free PMC article. No abstract available.
Abstract
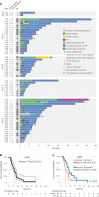
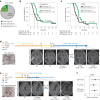
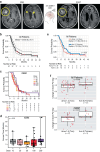
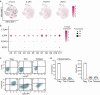
Chimeric antigen receptor T cell (CAR-T) therapy is an emerging strategy to improve treatment outcomes for recurrent high-grade glioma, a cancer that responds poorly to current therapies. Here we report a completed phase I trial evaluating IL-13Rα2-targeted CAR-T cells in 65 patients with recurrent high-grade glioma, the majority being recurrent glioblastoma (rGBM). Primary objectives were safety and feasibility, maximum tolerated dose/maximum feasible dose and a recommended phase 2 dose plan. Secondary objectives included overall survival, disease response, cytokine dynamics and tumor immune contexture biomarkers. This trial evolved to evaluate three routes of locoregional T cell administration (intratumoral (ICT), intraventricular (ICV) and dual ICT/ICV) and two manufacturing platforms, culminating in arm 5, which utilized dual ICT/ICV delivery and an optimized manufacturing process. Locoregional CAR-T cell administration was feasible and well tolerated, and as there were no dose-limiting toxicities across all arms, a maximum tolerated dose was not determined. Probable treatment-related grade 3+ toxicities were one grade 3 encephalopathy and one grade 3 ataxia. A clinical maximum feasible dose of 200 × 106 CAR-T cells per infusion cycle was achieved for arm 5; however, other arms either did not test or achieve this dose due to manufacturing feasibility. A recommended phase 2 dose will be refined in future studies based on data from this trial. Stable disease or better was achieved in 50% (29/58) of patients, with two partial responses, one complete response and a second complete response after additional CAR-T cycles off protocol. For rGBM, median overall survival for all patients was 7.7 months and for arm 5 was 10.2 months. Central nervous system increases in inflammatory cytokines, including IFNγ, CXCL9 and CXCL10, were associated with CAR-T cell administration and bioactivity. Pretreatment intratumoral CD3 T cell levels were positively associated with survival. These findings demonstrate that locoregional IL-13Rα2-targeted CAR-T therapy is safe with promising clinical activity in a subset of patients. ClinicalTrials.gov Identifier: NCT02208362 .
© 2024. The Author(s).
Conflict of interest statement
C.E.B. and S.J.F. report personal fees, patent royalties and research support from Mustang Bio during the conduct of the study, as well as patent royalties from Chimeric Therapeutics outside the submitted work. C.E.B., S.J.F. and B.B. also have a patent for CAR-T cell delivery pending and with royalties payable from Mustang Bio. N.E.B. receives compensation from DeepCell. The other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

