Exploring Aeromonas dhakensis in Aldabra giant tortoises: a debut report and genetic characterization
- PMID: 38454361
- PMCID: PMC10921707
- DOI: 10.1186/s12866-024-03203-w
Exploring Aeromonas dhakensis in Aldabra giant tortoises: a debut report and genetic characterization
Abstract
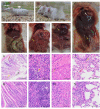
Aeromonas dhakensis (A. dhakensis) is becoming an emerging pathogen worldwide, with an increasingly significant role in animals and human health. It is a ubiquitous bacteria found in terrestrial and aquatic milieus. However, there have been few reports of reptile infections. In this study, a bacterial strain isolated from a dead Aldabra giant tortoise was identified as A. dhakensis HN-1 through clinical observation, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF/MS), and gene sequencing analysis. Subsequently, to evaluate its pathogenicity, the detection of virulence genes and mice infection experiments were performed. A. dhakensis HN-1 was found to contain seven virulence genes, including alt, ela, lip, act, aerA, fla, and hlyA. Mice infected with A. dhakensis HN-1 exhibited hemorrhage of varying degrees in multiple organs. The half-maximal lethal dose (LD50) value of A. dhakensis HN-1 for mice was estimated to be 2.05 × 107 colony forming units (CFU)/mL. The antimicrobial susceptibility test revealed that A. dhakensis HN-1 was resistant to amoxicillin, penicillin, ampicillin and erythromycin. This is the first report of A. dhakensis in Aldabra giant tortoises, expanding the currently known host spectrum. Our findings emphasize the need for One Health surveillance and extensive research to reduce the spread of A. dhakensis across the environment, humans, and animals.
Keywords: Aeromonas dhakensis; Aldabra giant tortoise; Multidrug resistance; Pathogenicity; Public health.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- World Health Organization. Zoonoses. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/zoonoses.
-
- Rusiñol M, Hundesa A, Cárdenas-Youngs Y, Fernández-Bravo A, Pérez-Cataluña A, Moreno-Mesonero L, Girones R, et al. Microbiological contamination of conventional and reclaimed irrigation water: evaluation and management measures. Sci Total Environ. 2020;710:136298. doi: 10.1016/j.scitotenv.2019.136298. - DOI - PubMed
-
- Pablos M, Remacha MA, Rodríguez-Calleja JM, Santos JA, Otero A, García-López ML. Identity, virulence genes, and clonal relatedness of Aeromonas isolates from patients with diarrhea and drinking water. Eur J Clin Microbiol Infect Dis. 2010;29(9):1163–72. doi: 10.1007/s10096-010-0982-3. - DOI - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

