TMEM160 promotes tumor immune evasion and radiotherapy resistance via PD-L1 binding in colorectal cancer
- PMID: 38454413
- PMCID: PMC10921666
- DOI: 10.1186/s12964-024-01541-w
TMEM160 promotes tumor immune evasion and radiotherapy resistance via PD-L1 binding in colorectal cancer
Abstract
Background: The effectiveness of anti-programmed cell death protein 1(PD-1)/programmed cell death 1 ligand 1(PD-L1) therapy in treating certain types of cancer is associated with the level of PD-L1. However, this relationship has not been observed in colorectal cancer (CRC), and the underlying regulatory mechanism of PD-L1 in CRC remains unclear.
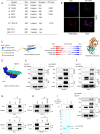
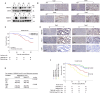
Methods: Binding of TMEM160 to PD-L1 was determined by co-immunoprecipitation (Co-IP) and GST pull-down assay.The ubiquitination levels of PD-L1 were verified using the ubiquitination assay. Phenotypic experiments were conducted to assess the role of TMEM160 in CRC cells. Animal models were employed to investigate how TMEM160 contributes to tumor growth.The expression and clinical significance of TMEM160 and PD-L1 in CRC tissues were evaluated by immunohistochemistry(IHC).
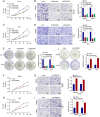
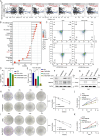
Results: In our study, we made a discovery that TMEM160 interacts with PD-L1 and plays a role in stabilizing its expression within a CRC model. Furthermore, we demonstrated that TMEM160 hinders the ubiquitination-dependent degradation of PD-L1 by competing with SPOP for binding to PD-L1 in CRC cells. Regarding functionality, the absence of TMEM160 significantly inhibited the proliferation, invasion, metastasis, clonogenicity, and radioresistance of CRC cells, while simultaneously enhancing the cytotoxic effect of CD8 + T cells on tumor cells. Conversely, the upregulation of TMEM160 substantially increased these capabilities. In severely immunodeficient mice, tumor growth derived from lentiviral vector shTMEM160 cells was lower compared with that derived from shNC control cells. Furthermore, the downregulation of TMEM160 significantly restricted tumor growth in immune-competent BALB/c mice. In clinical samples from patients with CRC, we observed a strong positive correlation between TMEM160 expression and PD-L1 expression, as well as a negative correlation with CD8A expression. Importantly, patients with high TMEM160 expression exhibited a worse prognosis compared with those with low or no TMEM160 expression.
Conclusions: Our study reveals that TMEM160 inhibits the ubiquitination-dependent degradation of PD-L1 that is mediated by SPOP, thereby stabilizing PD-L1 expression to foster the malignant progress, radioresistance, and immune evasion of CRC cells. These findings suggest that TMEM160 holds potential as a target for the treatment of patients with CRC.
Keywords: Colorectal cancer; PD-L1; SPOP; TMEM160.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Aldehyde Dehydrogenase 2 Mediates Alcohol-Induced Colorectal Cancer Immune Escape through Stabilizing PD-L1 Expression.Adv Sci (Weinh). 2021 Mar 24;8(10):2003404. doi: 10.1002/advs.202003404. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026438 Free PMC article.
-
N6-methyladenosine-modified circIGF2BP3 inhibits CD8+ T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer.Mol Cancer. 2021 Aug 20;20(1):105. doi: 10.1186/s12943-021-01398-4. Mol Cancer. 2021. PMID: 34416901 Free PMC article.
-
Targeting Nuclear Receptor Coactivator SRC-1 Prevents Colorectal Cancer Immune Escape by Reducing Transcription and Protein Stability of PD-L1.Adv Sci (Weinh). 2024 Sep;11(33):e2310037. doi: 10.1002/advs.202310037. Epub 2024 Jul 2. Adv Sci (Weinh). 2024. PMID: 38953362 Free PMC article.
-
A Systematic Review of the Tumor-Infiltrating CD8+ T-Cells/PD-L1 Axis in High-Grade Glial Tumors: Toward Personalized Immuno-Oncology.Front Immunol. 2021 Sep 17;12:734956. doi: 10.3389/fimmu.2021.734956. eCollection 2021. Front Immunol. 2021. PMID: 34603316 Free PMC article.
-
Unveiling the immune symphony: decoding colorectal cancer metastasis through immune interactions.Front Immunol. 2024 Feb 13;15:1362709. doi: 10.3389/fimmu.2024.1362709. eCollection 2024. Front Immunol. 2024. PMID: 38415252 Free PMC article. Review.
Cited by
-
The complex interplay of tumor-infiltrating cells in driving therapeutic resistance pathways.Cell Commun Signal. 2024 Aug 19;22(1):405. doi: 10.1186/s12964-024-01776-7. Cell Commun Signal. 2024. PMID: 39160622 Free PMC article. Review.
-
TMEM160 inhibits KEAP1 to suppress ferroptosis and induce chemoresistance in gastric cancer.Cell Death Dis. 2025 Apr 13;16(1):287. doi: 10.1038/s41419-025-07621-0. Cell Death Dis. 2025. PMID: 40223081 Free PMC article.
-
HERV Modulation in Colorectal Carcinoma Patients: A Snapshot of Endogenous Retroviral Transcriptome.J Med Virol. 2025 Mar;97(3):e70249. doi: 10.1002/jmv.70249. J Med Virol. 2025. PMID: 39992019 Free PMC article.
-
TMEM160 Promotes Tumor Growth in Lung Adenocarcinoma and Cervical Adenocarcinoma Cell Lines.Int J Mol Sci. 2025 Jan 27;26(3):1097. doi: 10.3390/ijms26031097. Int J Mol Sci. 2025. PMID: 39940865 Free PMC article.
-
Post-translational modifications of cancer immune checkpoints: mechanisms and therapeutic strategies.Mol Cancer. 2025 Jul 8;24(1):193. doi: 10.1186/s12943-025-02397-5. Mol Cancer. 2025. PMID: 40629335 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 20202BABL216050/Jiangxi Provincial Department of Science and Technology
- 20232BAB216080/Jiangxi Provincial Department of Science and Technology
- 82160469/National Natural Science Foundation of China
- 82160459/National Natural Science Foundation of China
- 82260491/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

