Regulation of cancer stem cells by CXCL1, a chemokine whose secretion is controlled by MCM2
- PMID: 38454443
- PMCID: PMC10921750
- DOI: 10.1186/s12885-024-12085-0
Regulation of cancer stem cells by CXCL1, a chemokine whose secretion is controlled by MCM2
Abstract
Background: A high expression pattern of minichromosome maintenance 2 (MCM2) has been observed in various cancers. MCM2 is a protein involved in the cell cycle and plays a role in cancer growth and differentiation by binding to six members of the MCM subfamily. The MCM protein family includes MCM2 through MCM7.
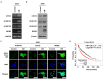
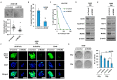
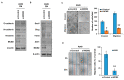
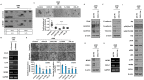
Methods: MCM2 has shown high expression in both lung cancer stem cells (LCSCs) and glioma stem cells (GSCs). We investigated the characteristics of CSCs and the regulation of the epithelial-to-mesenchymal transition (EMT) phenomenon in LCSCs and GSCs by MCM2. Additionally, we explored secreted factors regulated by MCM2.
Results: There was a significant difference in survival rates between lung cancer patients and brain cancer patients based on MCM2 expression. MCM2 was found to regulate both markers and regulatory proteins in LCSCs. Moreover, MCM2 is thought to be involved in cancer metastasis by regulating cell migration and invasion, not limited to lung cancer but also identified in glioma. Among chemokines, chemokine (C-X-C motif) ligand 1 (CXCL1) was found to be regulated by MCM2.
Conclusions: MCM2 not only participates in the cell cycle but also affects cancer cell growth by regulating the external microenvironment to create a favorable environment for cells. MCM2 is highly expressed in malignant carcinomas, including CSCs, and contributes to the malignancy of various cancers. Therefore, MCM2 may represent a crucial target for cancer therapeutics.
Keywords: CXCL1; Cancer stem cells; Chemokine; EMT; MCM2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

