Exposure to high dose of polystyrene nanoplastics causes trophoblast cell apoptosis and induces miscarriage
- PMID: 38454452
- PMCID: PMC10921758
- DOI: 10.1186/s12989-024-00574-w
Exposure to high dose of polystyrene nanoplastics causes trophoblast cell apoptosis and induces miscarriage
Abstract
Background: With rapid increase in the global use of various plastics, microplastics (MPs) and nanoplastics (NPs) pollution and their adverse health effects have attracted global attention. MPs have been detected out in human body and both MPs and NPs showed female reproductive toxicological effects in animal models. Miscarriage (abnormal early embryo loss), accounting for 15-25% pregnant women worldwide, greatly harms human reproduction. However, the adverse effects of NPs on miscarriage have never been explored.
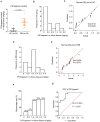
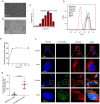
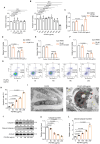
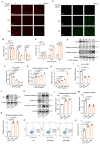
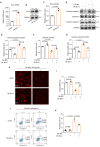
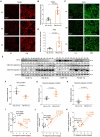
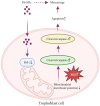
Results: In this study, we identified that polystyrene (PS) plastics particles were present in women villous tissues. Their levels were higher in villous tissues of unexplained recurrent miscarriage (RM) patients vs. healthy control (HC) group. Furthermore, mouse assays further confirmed that exposure to polystyrene nanoplastics (PS-NPs, 50 nm in diameter, 50 or 100 mg/kg) indeed induced miscarriage. In mechanism, PS-NPs exposure (50, 100, 150, or 200 µg/mL) increased oxidative stress, decreased mitochondrial membrane potential, and increased apoptosis in human trophoblast cells by activating Bcl-2/Cleaved-caspase-2/Cleaved-caspase-3 signaling through mitochondrial pathway. The alteration in this signaling was consistent in placental tissues of PS-NPs-exposed mouse model and in villous tissues of unexplained RM patients. Supplement with Bcl-2 could efficiently suppress apoptosis in PS-NPs-exposed trophoblast cells and reduce apoptosis and alleviate miscarriage in PS-NPs-exposed pregnant mouse model.
Conclusions: Exposure to PS-NPs activated Bcl-2/Cleaved-caspase-2/Cleaved-caspase-3, leading to excessive apoptosis in human trophoblast cells and in mice placental tissues, further inducing miscarriage.
Keywords: Apoptosis; Miscarriage; Polystyrene nanoplastics; Trophoblast.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Plastics-the. Facts 2021—An Analysis of European Plastics Production, Demand and Waste Data.
-
- Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M, et al. Are we speaking the same Language? Recommendations for a definition and categorization Framework for plastic debris. Environ Sci Technol. 2019;53(3):1039–47. doi: 10.1021/acs.est.8b05297. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

