Comparative analysis of the vaginal bacteriome and virome in healthy women living in high-altitude and sea-level areas
- PMID: 38454476
- PMCID: PMC10918948
- DOI: 10.1186/s40001-023-01391-1
Comparative analysis of the vaginal bacteriome and virome in healthy women living in high-altitude and sea-level areas
Abstract
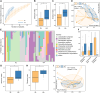
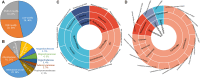
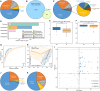
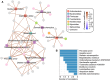
The vaginal microbiota plays an important role in the health of the female reproductive tract and is closely associated with various pregnancy outcomes and sexually transmitted diseases. Plenty of internal and external factors have strong influence on the changes in a woman's vaginal microbiome. However, the effect of a high-altitude on female vaginal microbiota has not been described. In this study, we characterized the vaginal bacteriome and virome of 13 and 34 healthy women living in high-altitude and sea-level areas, using whole-metagenome shotgun sequencing of their vaginal mucus samples. The results revealed that the vaginal bacteriomes of high-altitude individuals are featured by a significant increase of species diversity, depletion of Lactobacillus crispatus, and more abundant of some anaerobic bacteria, such as Chlamydia trachomatis, Mageeibacillus indolicus, Dialister micraerophilus, and Sneathia amnii). In addition, the vagina samples of sea-level subjects harbor more Lactobacillus strains, whereas the anaerobic bacteroidetes strains mostly appeared in high-altitude subjects. Identified and assembled 191 virus operational taxonomic units (vOTUs), there were significant differences in the abundance of 107 vOTUs between the two groups. Together, the results of this study raised the understanding of bacteriome and virome in the vagina of women at different elevations, and demonstrated that the vaginal microbiome is related to the high-altitude geographic adaptation.
Keywords: Bacteriome; High-altitude area; Vaginal microbiome; Virome; Whole-metagenome shotgun sequencing.
© 2024. The Author(s).
Conflict of interest statement
This manuscript has not been published elsewhere in whole or in part, and has been read and approved by all authors. All authors declare no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

