Macrophage-derived exosomal HMGB3 regulates silica-induced pulmonary inflammation by promoting M1 macrophage polarization and recruitment
- PMID: 38454505
- PMCID: PMC10918916
- DOI: 10.1186/s12989-024-00568-8
Macrophage-derived exosomal HMGB3 regulates silica-induced pulmonary inflammation by promoting M1 macrophage polarization and recruitment
Abstract
Background: Chronic inflammation and fibrosis are characteristics of silicosis, and the inflammatory mediators involved in silicosis have not been fully elucidated. Recently, macrophage-derived exosomes have been reported to be inflammatory modulators, but their role in silicosis has not been explored. The purpose of the present study was to investigate the role of macrophage-derived exosomal high mobility group box 3 (HMGB3) in silica-induced pulmonary inflammation.
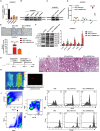
Methods: The induction of the inflammatory response and the recruitment of monocytes/macrophages were evaluated by immunofluorescence, flow cytometry and transwell assays. The expression of inflammatory cytokines was examined by RT-PCR and ELISA, and the signalling pathways involved were examined by western blot analysis.
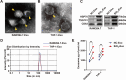
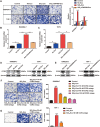
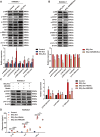
Results: HMGB3 expression was increased in exosomes derived from silica-exposed macrophages. Exosomal HMGB3 significantly upregulated the expression of inflammatory cytokines, activated the STAT3/MAPK (ERK1/2 and p38)/NF-κB pathways in monocytes/macrophages, and promoted the migration of these cells by CCR2.
Conclusions: Exosomal HMGB3 is a proinflammatory modulator of silica-induced inflammation that promotes the inflammatory response and recruitment of monocytes/macrophages by regulating the activation of the STAT3/MAPK/NF-κB/CCR2 pathways.
Keywords: HMGB3; Inflammation; Macrophage polarization; Silicosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Fermented Cordyceps Powder alleviates silica-induced inflammation and fibrosis by inhibiting M1 macrophage polarization via the HMGB1-TLR4-NF-κB pathway.J Ethnopharmacol. 2025 Apr 9;345:119631. doi: 10.1016/j.jep.2025.119631. Epub 2025 Mar 14. J Ethnopharmacol. 2025. PMID: 40090429
-
Mefunidone alleviates silica-induced inflammation and fibrosis by inhibiting the TLR4-NF-κB/MAPK pathway and attenuating pyroptosis in murine macrophages.Biomed Pharmacother. 2024 Sep;178:117216. doi: 10.1016/j.biopha.2024.117216. Epub 2024 Aug 2. Biomed Pharmacother. 2024. PMID: 39096618
-
Silica particles disorganize the polarization of pulmonary macrophages in mice.Ecotoxicol Environ Saf. 2020 Apr 15;193:110364. doi: 10.1016/j.ecoenv.2020.110364. Epub 2020 Feb 27. Ecotoxicol Environ Saf. 2020. PMID: 32114243
-
[Research progress of macrophage polarization in silicosis fibrosis].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2024 Apr 20;42(4):315-320. doi: 10.3760/cma.j.cn121094-20230306-00067. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2024. PMID: 38678001 Review. Chinese.
-
A mechanistic review-regulation of silica-induced pulmonary inflammation by IL-10 and exacerbation by Type I IFN.Inhal Toxicol. 2025 Feb;37(2):59-73. doi: 10.1080/08958378.2025.2465378. Epub 2025 Feb 16. Inhal Toxicol. 2025. PMID: 39955624 Review.
Cited by
-
Biomimetic ECM nerve guidance conduit with dynamic 3D interconnected porous network and sustained IGF-1 delivery for enhanced peripheral nerve regeneration and immune modulation.Mater Today Bio. 2024 Dec 12;30:101403. doi: 10.1016/j.mtbio.2024.101403. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39790488 Free PMC article.
-
Long COVID in Elderly COPD Patients: Clinical Features, Pulmonary Function Decline, and Proteomic Insights.Int J Chron Obstruct Pulmon Dis. 2025 Jul 8;20:2337-2347. doi: 10.2147/COPD.S520300. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40655185 Free PMC article.
-
Hsa_circ_0004662 Accelerates the Progression of Ulcerative Colitis via the microRNA-532/HMGB3 Signalling Axis.J Cell Mol Med. 2025 Mar;29(6):e70430. doi: 10.1111/jcmm.70430. J Cell Mol Med. 2025. PMID: 40099942 Free PMC article.
-
Structure and Functions of HMGB3 Protein.Int J Mol Sci. 2024 Jul 12;25(14):7656. doi: 10.3390/ijms25147656. Int J Mol Sci. 2024. PMID: 39062899 Free PMC article. Review.
References
-
- China NHCotPsRo. The Statistical Bulletin of China’s Health Service Development in 2021 2022 [Available from: http://www.gov.cn/xinwen/2022-07/12/content_5700670.htm.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

