Targeting lipid nanoparticles to the blood-brain barrier to ameliorate acute ischemic stroke
- PMID: 38454606
- PMCID: PMC11081939
- DOI: 10.1016/j.ymthe.2024.03.004
Targeting lipid nanoparticles to the blood-brain barrier to ameliorate acute ischemic stroke
Abstract
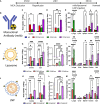
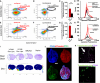
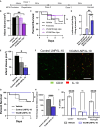
Effective delivery of mRNA or small molecule drugs to the brain is a significant challenge in developing treatment for acute ischemic stroke (AIS). To address the problem, we have developed targeted nanomedicine to increase drug concentrations in endothelial cells of the blood-brain barrier (BBB) of the injured brain. Inflammation during ischemic stroke causes continuous neuronal death and an increase in the infarct volume. To enable targeted delivery to the inflamed BBB, we conjugated lipid nanocarriers (NCs) with antibodies that bind cell adhesion molecules expressed at the BBB. In the transient middle cerebral artery occlusion mouse model, NCs targeted to vascular cellular adhesion molecule-1 (VCAM) achieved the highest level of brain delivery, nearly two orders of magnitude higher than untargeted ones. VCAM-targeted lipid nanoparticles with luciferase-encoding mRNA and Cre-recombinase showed selective expression in the ischemic brain. Anti-inflammatory drugs administered intravenously after ischemic stroke reduced cerebral infarct volume by 62% (interleukin-10 mRNA) or 35% (dexamethasone) only when they were encapsulated in VCAM-targeted NCs. Thus, VCAM-targeted lipid NCs represent a new platform for strongly concentrating drugs within the compromised BBB of penumbra, thereby ameliorating AIS.
Keywords: drug delivery; drug targeting; ischemic stroke; lipid nanoparticles; neurovascular inflammation.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests J.N., P.M.G., V.V.S., H.P., D.W., J.S.B., V.R.M., and O.A.M-C. have pending patent applications fully disclosed by the University of Pennsylvania.
Figures

Update of
-
Targeting lipid nanoparticles to the blood brain barrier to ameliorate acute ischemic stroke.bioRxiv [Preprint]. 2023 Jun 13:2023.06.12.544645. doi: 10.1101/2023.06.12.544645. bioRxiv. 2023. Update in: Mol Ther. 2024 May 1;32(5):1344-1358. doi: 10.1016/j.ymthe.2024.03.004. PMID: 37398465 Free PMC article. Updated. Preprint.
References
-
- Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. - DOI - PubMed
-
- Albers G.W., Marks M.P., Kemp S., Christensen S., Tsai J.P., Ortega-Gutierrez S., McTaggart R.A., Torbey M.T., Kim-Tenser M., Leslie-Mazwi T., et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL160694/HL/NHLBI NIH HHS/United States
- R01 HL143806/HL/NHLBI NIH HHS/United States
- K08 HL138269/HL/NHLBI NIH HHS/United States
- R01 NS131279/NS/NINDS NIH HHS/United States
- R01 HL153510/HL/NHLBI NIH HHS/United States
- R01 HL157189/HL/NHLBI NIH HHS/United States
- R01 HL155106/HL/NHLBI NIH HHS/United States
- T32 GM008076/GM/NIGMS NIH HHS/United States
- R01 HL164594/HL/NHLBI NIH HHS/United States
- F31 HL154662/HL/NHLBI NIH HHS/United States
- R21 AI166778/AI/NIAID NIH HHS/United States
- R01 HL128398/HL/NHLBI NIH HHS/United States
- R41 NS130812/NS/NINDS NIH HHS/United States
- R00 HL153696/HL/NHLBI NIH HHS/United States
- K99 HL153696/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

