Perioperative immunotherapy for stage II-III non-small cell lung cancer: a meta-analysis base on randomized controlled trials
- PMID: 38454928
- PMCID: PMC10917905
- DOI: 10.3389/fonc.2024.1351359
Perioperative immunotherapy for stage II-III non-small cell lung cancer: a meta-analysis base on randomized controlled trials
Abstract
Background: In recent years, we have observed the pivotal role of immunotherapy in improving survival for patients with non-small cell lung cancer (NSCLC). However, the effectiveness of immunotherapy in the perioperative (neoadjuvant + adjuvant) treatment of resectable NSCLC remains uncertain. We conducted a comprehensive analysis of its antitumor efficacy and adverse effects (AEs) by pooling data from the KEYNOTE-671, NADIM II, and AEGEAN clinical trials.
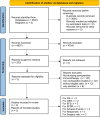
Methods: For eligible studies, we searched seven databases. The randomized controlled trials (RCTs) pertaining to the comparative analysis of combination neoadjuvant platinum-based chemotherapy plus perioperative immunotherapy (PIO) versus perioperative placebo (PP) were included. Primary endpoints were overall survival (OS) and event-free survival (EFS). Secondary endpoints encompassed drug responses, AEs, and surgical outcomes.
Results: Three RCTs (KEYNOTE-671, NADIM II, and AEGEAN) were included in the final analysis. PIO group (neoadjuvant platinum-based chemotherapy plus perioperative immunotherapy) exhibited superior efficacy in OS (hazard ratio [HR]: 0.63 [0.49-0.81]), EFS (HR: 0.61 [0.52, 0.72]), objective response rate (risk ratio [RR]: 2.21 [1.91, 2.54]), pathological complete response (RR: 4.36 [3.04, 6.25]), major pathological response (RR: 2.79 [2.25, 3.46]), R0 resection rate (RR: 1.13 [1.00, 1.26]) and rate of adjuvant treatment (RR: 1.08 [1.01, 1.15]) compared with PP group (neoadjuvant platinum-based chemotherapy plus perioperative placebo). In the subgroup analysis, EFS tended to favor the PIO group in almost all subgroups. BMI (>25), T stage (IV), N stage (N1-N2) and pathological response (with pathological complete response) were favorable factors in the PIO group. In the safety assessment, the PIO group exhibited higher rates of serious AEs (28.96% vs. 23.51%) and AEs leading to treatment discontinuation (12.84% vs. 5.81%). Meanwhile, although total adverse events, grade 3-5 adverse events, and fatal adverse events tended to favor the PP group, the differences were not statistically significant.
Conclusion: PIO appears to be superior to PP for resectable stage II-III NSCLC, demonstrating enhanced survival and pathological responses. However, its elevated adverse event (AE) rate warrants careful consideration.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails, identifier CRD42023487475.
Keywords: adjuvant; immunotherapy; meta-analysis; neoadjuvant; non-small cell lung cancer; surgery.
Copyright © 2024 Yu, Fu, Li, Wu, Yu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Efficacy and safety of neoadjuvant immunotherapy plus chemotherapy followed by adjuvant immunotherapy in resectable non-small cell lung cancer: a meta-analysis of phase 3 clinical trials.Front Immunol. 2024 Apr 5;15:1359302. doi: 10.3389/fimmu.2024.1359302. eCollection 2024. Front Immunol. 2024. PMID: 38646542 Free PMC article.
-
Efficacy and safety of perioperative, neoadjuvant, or adjuvant immunotherapy alone or in combination with chemotherapy in early-stage non-small cell lung cancer: a systematic review and meta-analysis of randomized clinical trials.Ther Adv Med Oncol. 2024 Oct 4;16:17588359241284929. doi: 10.1177/17588359241284929. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39376583 Free PMC article.
-
Perioperative or neo/adjuvant chemoimmunotherapy versus chemotherapy for resectable non-small cell lung cancer: a systematic review and network meta-analysis.Syst Rev. 2025 Jan 24;14(1):24. doi: 10.1186/s13643-025-02767-6. Syst Rev. 2025. PMID: 39856765 Free PMC article.
-
Perioperative PD-1/PD-L1 inhibitors for resectable non-small cell lung cancer: A meta-analysis based on randomized controlled trials.PLoS One. 2024 Sep 23;19(9):e0310808. doi: 10.1371/journal.pone.0310808. eCollection 2024. PLoS One. 2024. PMID: 39312569 Free PMC article.
-
Efficacy and Safety of Perioperative Immunotherapy for Patients with Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis.Curr Oncol. 2025 Mar 20;32(3):184. doi: 10.3390/curroncol32030184. Curr Oncol. 2025. PMID: 40136388 Free PMC article.
Cited by
-
Perioperative Management of Non-Small Cell Lung Cancer in the Era of Immunotherapy.Cells. 2025 Jun 25;14(13):971. doi: 10.3390/cells14130971. Cells. 2025. PMID: 40643492 Free PMC article. Review.
-
A narrative review on perioperative systemic therapy in non-small cell lung cancer.Explor Target Antitumor Ther. 2024;5(4):931-954. doi: 10.37349/etat.2024.00256. Epub 2024 Jul 26. Explor Target Antitumor Ther. 2024. PMID: 39280253 Free PMC article. Review.
References
-
- Expert Consensus Panel. Kidane B, Bott M, Spicer J, Backhus L, Chaft J, et al. . The American Association for Thoracic Surgery (AATS) 2023 Expert Consensus Document: Staging and multidisciplinary management of patients with early-stage non-small cell lung cancer. J Thorac Cardiovasc Surg. (2023) 166:637–54. doi: 10.1016/j.jtcvs.2023.04.039. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

