Safety and Effectiveness of a Biosimilar Recombinant Human Growth Hormone in Children Requiring Growth Hormone Treatment: Analysis of Final Data from PATRO Children, an International, Post-Marketing Surveillance Study
- PMID: 38454934
- PMCID: PMC10918591
- DOI: 10.2147/DDDT.S440009
Safety and Effectiveness of a Biosimilar Recombinant Human Growth Hormone in Children Requiring Growth Hormone Treatment: Analysis of Final Data from PATRO Children, an International, Post-Marketing Surveillance Study
Abstract
Purpose: Omnitrope® (somatropin) was approved as a biosimilar recombinant human growth hormone (rhGH) in 2006. Here, we report final data from the PAtients TReated with Omnitrope® (PATRO) Children study, a post-marketing surveillance study designed to monitor the long-term safety and effectiveness of this treatment in pediatric patients.
Methods: The study population included all pediatric patients treated with Omnitrope® (biosimilar rhGH), administered via daily injection, in routine clinical practice. The primary objective was to assess long-term safety, with effectiveness assessed as a secondary objective.
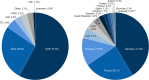
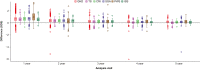
Results: In total, 7359 patients were enrolled and treated in the PATRO Children study; 86.0% were treatment-naïve at baseline. Growth hormone deficiency was the most frequent indication (57.9%), followed by patients born small for gestational age (SGA; 26.6%). The mean (SD) duration of exposure to biosimilar rhGH was 3.66 years (2.39). A total of 16,628 adverse events (AEs) were reported in 3981 (54.1%) patients, most of which were mild/moderate. AEs suspected to be treatment related occurred in 8.3% of patients, most frequently headache (1.6%), injection-site pain (1.1%), or injection-site hematoma (1.1%). The incidence rate (IR) of type 2 diabetes mellitus was 0.11 per 1000 person-years (PY) across all patients, and 0.13 per 1000 PY in patients born SGA. The IR of newly diagnosed primary malignancies was 0.22 per 1000 PY across all patients. In the 6589 patients included in the effectiveness population, a sustained catch-up growth was observed across all indications. After 5 years of treatment, height SDS increased from baseline by a median (range) of +1.79 (-3.7 to 6.2) in treatment-naïve patients and +0.73 (-1.4 to 3.7) in pretreated patients.
Conclusion: This final analysis of the PATRO Children study indicates that biosimilar rhGH is well tolerated and effective in real-world clinical practice. These data are consistent with the well-characterized safety profile of rhGH treatment in pediatric patients.
Keywords: Omnitrope; PATRO Children; growth hormone; growth hormone deficiency; pediatrics; small for gestational age.
© 2024 Loche et al.
Conflict of interest statement
Sandro Loche is a member of the PATRO Children Advisory Board and the PATRO Children Global Steering Committee, and has received advisory board and lecture fees from Sandoz, Merck Serono, Ipsen, and Pfizer. Shankar Kanumakala has received support to attend scientific meetings from Sandoz in previous years, and is a member of the PATRO Children Global Steering Committee. Philippe Backeljauw is a member of the PATRO Children Advisory Board, and has received advisory board, consultancy, and lecture fees from Novartis/Sandoz, BioMarin, Ascendis, Tolmar, Cavalry Biosciences, Novo Nordisk, and Ipsen. Karl Otfried Schwab is a member of the German PATRO Children Advisory Board and the PATRO Children Advisory Board, and has received advisory board and lecture fees from Akcea, Alexion, Merck Healthcare, Novartis, Pfizer, and Sanofi-Aventis. Alfonso M Lechuga-Sancho is a member of an external advisory board for Sandoz and Ipsen, and has received research grants from Abbott, Medtronic, Menarini, Merck Serono, Sanofi, and Novo Nordisk. Altaher Esmael and Markus Zabransky are employees of HEXAL AG (a Sandoz company). Dragan Urosevic is an employee of Novartis Sandoz Biopharmaceutical AG. Anca Boldea was an employee of HEXAL AG (a Sandoz company) when the study and manuscript development were undertaken. The authors report no other conflicts of interest in this work.
Figures



References
-
- European Medicines Agency [Internet]. Omnitrope® summary of product characteristics, updated 2023; 2008. Available from: https://www.ema.europa.eu/en/documents/product-information/omnitrope-epa.... Accessed June 30, 2023.
-
- European Medicines Agency [Internet]. Omnitrope® European public assessment report (EPAR), updated 2018; 2008. Available from: https://www.ema.europa.eu/en/documents/overview/omnitrope-epar-summary-p.... Accessed June 30, 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

