A genetic optimization strategy with generality in asymmetric organocatalysis as a primary target
- PMID: 38455002
- PMCID: PMC10915838
- DOI: 10.1039/d3sc06208b
A genetic optimization strategy with generality in asymmetric organocatalysis as a primary target
Abstract
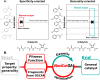
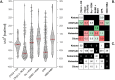
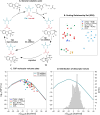
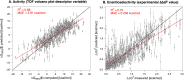
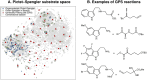
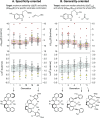
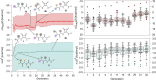
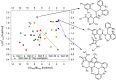
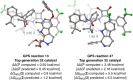
A catalyst possessing a broad substrate scope, in terms of both turnover and enantioselectivity, is sometimes called "general". Despite their great utility in asymmetric synthesis, truly general catalysts are difficult or expensive to discover via traditional high-throughput screening and are, therefore, rare. Existing computational tools accelerate the evaluation of reaction conditions from a pre-defined set of experiments to identify the most general ones, but cannot generate entirely new catalysts with enhanced substrate breadth. For these reasons, we report an inverse design strategy based on the open-source genetic algorithm NaviCatGA and on the OSCAR database of organocatalysts to simultaneously probe the catalyst and substrate scope and optimize generality as a primary target. We apply this strategy to the Pictet-Spengler condensation, for which we curate a database of 820 reactions, used to train statistical models of selectivity and activity. Starting from OSCAR, we define a combinatorial space of millions of catalyst possibilities, and perform evolutionary experiments on a diverse substrate scope that is representative of the whole chemical space of tetrahydro-β-carboline products. While privileged catalysts emerge, we show how genetic optimization can address the broader question of generality in asymmetric synthesis, extracting structure-performance relationships from the challenging areas of chemical space.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Strassfeld D. A. Algera R. F. Wickens Z. K. Jacobsen E. N. A Case Study in Catalyst Generality: Simultaneous, Highly-Enantioselective Brønsted- and Lewis-Acid Mechanisms in Hydrogen-Bond-Donor Catalyzed Oxetane Openings. J. Am. Chem. Soc. 2021;143:9585–9594. doi: 10.1021/jacs.1c03992. - DOI - PMC - PubMed
-
- Gao X. Kagan H. B. One-pot multi-substrate screening in asymmetric catalysis. Chirality. 1998;10:120–124. doi: 10.1002/chir.19. - DOI
LinkOut - more resources
Full Text Sources

