Mapping of a hybrid insulin peptide in the inflamed islet β-cells from NOD mice
- PMID: 38455055
- PMCID: PMC10917911
- DOI: 10.3389/fimmu.2024.1348131
Mapping of a hybrid insulin peptide in the inflamed islet β-cells from NOD mice
Abstract
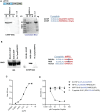
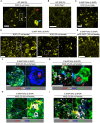
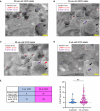
There is accumulating evidence that pathogenic T cells in T1D recognize epitopes formed by post-translational modifications of β-cell antigens, including hybrid insulin peptides (HIPs). The ligands for several CD4 T-cell clones derived from the NOD mouse are HIPs composed of a fragment of proinsulin joined to peptides from endogenous β-cell granule proteins. The diabetogenic T-cell clone BDC-6.9 reacts to a fragment of C-peptide fused to a cleavage product of pro-islet amyloid polypeptide (6.9HIP). In this study, we used a monoclonal antibody (MAb) to the 6.9HIP to determine when and where HIP antigens are present in NOD islets during disease progression and with which immune cells they associate. Immunogold labeling of the 6.9HIP MAb and organelle-specific markers for electron microscopy were employed to map the subcellular compartment(s) in which the HIP is localized within β-cells. While the insulin B9-23 peptide was present in nearly all islets, the 6.9HIP MAb stained infiltrated islets only in NOD mice at advanced stages of T1D development. Islets co-stained with the 6.9HIP MAb and antibodies to mark insulin, macrophages, and dendritic cells indicate that 6.9HIP co-localizes within insulin-positive β-cells as well as intra-islet antigen-presenting cells (APCs). In electron micrographs, the 6.9HIP co-localized with granule structures containing insulin alone or both insulin and LAMP1 within β-cells. Exposing NOD islets to the endoplasmic reticulum (ER) stress inducer tunicamycin significantly increased levels of 6.9HIP in subcellular fractions containing crinosomes and dense-core granules (DCGs). This work demonstrates that the 6.9HIP can be visualized in the infiltrated islets and suggests that intra-islet APCs may acquire and present HIP antigens within islets.
Keywords: NOD; antigen; autoimmune diabetes; hybrid insulin peptide; islets; post-translational modification; type 1 diabetes; β-cells.
Copyright © 2024 Wenzlau, Peterson, Vomund, DiLisio, Hohenstein, Haskins and Wan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse.J Autoimmun. 2017 Mar;78:11-18. doi: 10.1016/j.jaut.2016.10.007. Epub 2016 Oct 29. J Autoimmun. 2017. PMID: 27802879 Free PMC article.
-
Insulin B-chain hybrid peptides are agonists for T cells reactive to insulin B:9-23 in autoimmune diabetes.Front Immunol. 2022 Aug 10;13:926650. doi: 10.3389/fimmu.2022.926650. eCollection 2022. Front Immunol. 2022. PMID: 36032090 Free PMC article.
-
Proinsulin C-peptide is a major source of HLA-DQ8 restricted hybrid insulin peptides recognized by human islet-infiltrating CD4+ T cells.PNAS Nexus. 2024 Nov 1;3(11):pgae491. doi: 10.1093/pnasnexus/pgae491. eCollection 2024 Nov. PNAS Nexus. 2024. PMID: 39554513 Free PMC article.
-
The central role of antigen presentation in islets of Langerhans in autoimmune diabetes.Curr Opin Immunol. 2014 Feb;26:32-40. doi: 10.1016/j.coi.2013.10.011. Epub 2013 Nov 16. Curr Opin Immunol. 2014. PMID: 24556398 Free PMC article. Review.
-
Antigen presentation events during the initiation of autoimmune diabetes in the NOD mouse.J Autoimmun. 2016 Jul;71:19-25. doi: 10.1016/j.jaut.2016.03.007. Epub 2016 Mar 24. J Autoimmun. 2016. PMID: 27021276 Free PMC article. Review.
Cited by
-
Novel T-Cell Reactivities to Hybrid Insulin Peptides in Islet Autoantibody-Positive At-Risk Individuals.Diabetes. 2025 Jun 1;74(6):933-942. doi: 10.2337/db24-0611. Diabetes. 2025. PMID: 39820647
-
Dysfunctional β-cell autophagy induces β-cell stress and enhances islet immunogenicity.Front Immunol. 2025 Jan 29;16:1504583. doi: 10.3389/fimmu.2025.1504583. eCollection 2025. Front Immunol. 2025. PMID: 39944686 Free PMC article.
-
Crinophagic granules in pancreatic β cells contribute to mouse autoimmune diabetes by diversifying pathogenic epitope repertoire.Nat Commun. 2024 Sep 27;15(1):8318. doi: 10.1038/s41467-024-52619-5. Nat Commun. 2024. PMID: 39333495 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

